Brain metabolism and cerebrospinal fluid biomarkers profile of non-amnestic mild cognitive impairment in comparison to amnestic mild cognitive impairment and normal older subjects
- PMID: 26373380
- PMCID: PMC4572657
- DOI: 10.1186/s13195-015-0143-0
Brain metabolism and cerebrospinal fluid biomarkers profile of non-amnestic mild cognitive impairment in comparison to amnestic mild cognitive impairment and normal older subjects
Abstract
Introduction: Mild cognitive impairment (MCI) is classically considered a transitional stage between normal aging and dementia. Non-amnestic MCI (naMCI) patients, however, typically demonstrate cognitive deficits other than memory decline. Furthermore, as a group, naMCI have a lower rate of an eventual dementia diagnosis as compared to amnestic subtypes of MCI (aMCI). Unfortunately, studies investigating biomarker profiles of naMCI are scarce. The study objective was to investigate the regional brain glucose metabolism (rBGM) with [18F]FDG-PET and cerebrospinal fluid (CSF) biomarkers in subjects with naMCI as compared to a control group (CG) and aMCI subjects.
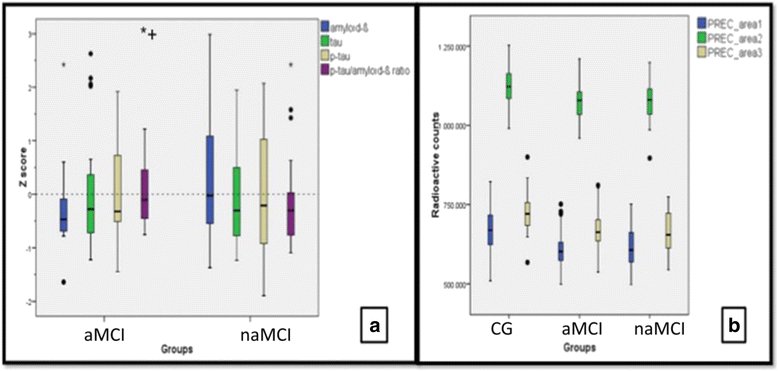
Methods: Ninety-five patients were included in three different groups: naMCI (N = 32), aMCI (N = 33) and CG (N = 30). Patients underwent brain MRI and [18F]FDG-PET. A subsample (naMCI = 26, aMCI = 28) also had an assessment of amyloid-β, tau, and phosphorylated tau levels in the CSF.
Results: Both MCI groups had lower rBGM in relation to the CG in the precuneus. Subjects with naMCI showed decreased right prefrontal metabolism as well as higher levels of CSF amyloid-β relative to aMCI subjects.
Conclusion: While amnestic MCI subjects showed a biomarker profile classically related to MCI due to Alzheimer's disease, naMCI patients illustrated a decrease in both prefrontal hypometabolism and higher CSF amyloid-β levels relative to the aMCI group. These biomarker findings indicate that naMCI is probably a heterogeneous group with similar precuneus hypometabolism compared to aMCI, but additional frontal hypometabolism and less amyloid-β deposition in the brain. Clinical follow-up and reappraisal of biomarkers of the naMCI group is needed to determine the outcome and probable etiological diagnosis.
Figures

References
-
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2014;256:240–6. doi: 10.1111/j.1365-2796.2004.01380.x. - DOI - PubMed
-
- Espinosa A, Alegret M, Valero S, Vinyes-Junqué G, Hernández I, Mauleón A, et al. A longitudinal follow-up of 550 mild cognitive impairment patients: evidence for large conversion to dementia rates and detection of major risk factors involved. J Alzheimers Dis. 2013;34:769–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

