WRKY Transcription Factors Phosphorylated by MAPK Regulate a Plant Immune NADPH Oxidase in Nicotiana benthamiana
- PMID: 26373453
- PMCID: PMC4815087
- DOI: 10.1105/tpc.15.00213
WRKY Transcription Factors Phosphorylated by MAPK Regulate a Plant Immune NADPH Oxidase in Nicotiana benthamiana
Abstract
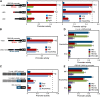
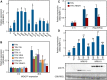
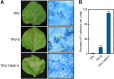
Pathogen attack sequentially confers pattern-triggered immunity (PTI) and effector-triggered immunity (ETI) after sensing of pathogen patterns and effectors by plant immune receptors, respectively. Reactive oxygen species (ROS) play pivotal roles in PTI and ETI as signaling molecules. Nicotiana benthamiana RBOHB, an NADPH oxidase, is responsible for both the transient PTI ROS burst and the robust ETI ROS burst. Here, we show that RBOHB transactivation mediated by MAPK contributes to R3a/AVR3a-triggered ETI (AVR3a-ETI) ROS burst. RBOHB is markedly induced during the ETI and INF1-triggered PTI (INF1-PTI), but not flg22-tiggered PTI (flg22-PTI). We found that the RBOHB promoter contains a functional W-box in the R3a/AVR3a and INF1 signal-responsive cis-element. Ectopic expression of four phospho-mimicking mutants of WRKY transcription factors, which are MAPK substrates, induced RBOHB, and yeast one-hybrid analysis indicated that these mutants bind to the cis-element. Chromatin immunoprecipitation assays indicated direct binding of the WRKY to the cis-element in plants. Silencing of multiple WRKY genes compromised the upregulation of RBOHB, resulting in impairment of AVR3a-ETI and INF1-PTI ROS bursts, but not the flg22-PTI ROS burst. These results suggest that the MAPK-WRKY pathway is required for AVR3a-ETI and INF1-PTI ROS bursts by activation of RBOHB.
© 2015 American Society of Plant Biologists. All rights reserved.
Figures







Similar articles
-
MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana.Plant Cell. 2008 May;20(5):1390-406. doi: 10.1105/tpc.107.055855. Epub 2008 May 30. Plant Cell. 2008. PMID: 18515503 Free PMC article.
-
PAMP-responsive ATL gene StRFP1 and its orthologue NbATL60 positively regulate Phytophthora infestans resistance in potato and Nicotiana benthamiana.Plant Sci. 2018 May;270:47-57. doi: 10.1016/j.plantsci.2018.01.016. Epub 2018 Feb 3. Plant Sci. 2018. PMID: 29576086
-
Phytophthora infestans RXLR-WY Effector AVR3a Associates with Dynamin-Related Protein 2 Required for Endocytosis of the Plant Pattern Recognition Receptor FLS2.PLoS One. 2015 Sep 8;10(9):e0137071. doi: 10.1371/journal.pone.0137071. eCollection 2015. PLoS One. 2015. PMID: 26348328 Free PMC article.
-
The PTI to ETI Continuum in Phytophthora-Plant Interactions.Front Plant Sci. 2020 Dec 17;11:593905. doi: 10.3389/fpls.2020.593905. eCollection 2020. Front Plant Sci. 2020. PMID: 33391306 Free PMC article. Review.
-
Plant immunity triggered by microbial molecular signatures.Mol Plant. 2010 Sep;3(5):783-93. doi: 10.1093/mp/ssq035. Epub 2010 Aug 16. Mol Plant. 2010. PMID: 20713980 Review.
Cited by
-
Genome-wide analysis and transcript profiling of PSKR gene family members in Oryza sativa.PLoS One. 2020 Jul 23;15(7):e0236349. doi: 10.1371/journal.pone.0236349. eCollection 2020. PLoS One. 2020. PMID: 32701993 Free PMC article.
-
Functional Analysis of MaWRKY24 in Transcriptional Activation of Autophagy-Related Gene 8f/g and Plant Disease Susceptibility to Soil-Borne Fusarium oxysporum f. sp. cubense.Pathogens. 2019 Nov 25;8(4):264. doi: 10.3390/pathogens8040264. Pathogens. 2019. PMID: 31775365 Free PMC article.
-
The Promoter Analysis of VvPR1 Gene: A Candidate Gene Identified through Transcriptional Profiling of Methyl Jasmonate Treated Grapevine (Vitis vinifera L.).Plants (Basel). 2022 Jun 9;11(12):1540. doi: 10.3390/plants11121540. Plants (Basel). 2022. PMID: 35736691 Free PMC article.
-
Tobacco Transcription Factor NtWRKY70b Facilitates Leaf Senescence via Inducing ROS Accumulation and Impairing Hydrogen Sulfide Biosynthesis.Int J Mol Sci. 2024 Mar 26;25(7):3686. doi: 10.3390/ijms25073686. Int J Mol Sci. 2024. PMID: 38612502 Free PMC article.
-
Transcriptome analysis reveals that exogenous ethylene activates immune and defense responses in a high late blight resistant potato genotype.Sci Rep. 2020 Dec 4;10(1):21294. doi: 10.1038/s41598-020-78027-5. Sci Rep. 2020. PMID: 33277549 Free PMC article.
References
-
- Adachi H., Yoshioka H. (2015). Kinase-mediated orchestration of NADPH oxidase in plant immunity. Brief. Funct. Genomics 14: 253–259. - PubMed
-
- Asai S., Ichikawa T., Nomura H., Kobayashi M., Kamiyoshihara Y., Mori H., Kadota Y., Zipfel C., Jones J.D.G., Yoshioka H. (2013). The variable domain of a plant calcium-dependent protein kinase (CDPK) confers subcellular localization and substrate recognition for NADPH oxidase. J. Biol. Chem. 288: 14332–14340. - PMC - PubMed
-
- Asai T., Tena G., Plotnikova J., Willmann M.R., Chiu W.L., Gomez-Gomez L., Boller T., Ausubel F.M., Sheen J. (2002). MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

