Two Essential Light Chains Regulate the MyoA Lever Arm To Promote Toxoplasma Gliding Motility
- PMID: 26374117
- PMCID: PMC4600101
- DOI: 10.1128/mBio.00845-15
Two Essential Light Chains Regulate the MyoA Lever Arm To Promote Toxoplasma Gliding Motility
Abstract
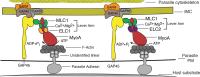
Key to the virulence of apicomplexan parasites is their ability to move through tissue and to invade and egress from host cells. Apicomplexan motility requires the activity of the glideosome, a multicomponent molecular motor composed of a type XIV myosin, MyoA. Here we identify a novel glideosome component, essential light chain 2 (ELC2), and functionally characterize the two essential light chains (ELC1 and ELC2) of MyoA in Toxoplasma. We show that these proteins are functionally redundant but are important for invasion, egress, and motility. Molecular simulations of the MyoA lever arm identify a role for Ca(2+) in promoting intermolecular contacts between the ELCs and the adjacent MLC1 light chain to stabilize this domain. Using point mutations predicted to ablate either the interaction with Ca(2+) or the interface between the two light chains, we demonstrate their contribution to the quality, displacement, and speed of gliding Toxoplasma parasites. Our work therefore delineates the importance of the MyoA lever arm and highlights a mechanism by which this domain could be stabilized in order to promote invasion, egress, and gliding motility in apicomplexan parasites.
Importance: Tissue dissemination and host cell invasion by apicomplexan parasites such as Toxoplasma are pivotal to their pathogenesis. Central to these processes is gliding motility, which is driven by an actomyosin motor, the MyoA glideosome. Others have demonstrated the importance of the MyoA glideosome for parasite motility and virulence in mice. Disruption of its function may therefore have therapeutic potential, and yet a deeper mechanistic understanding of how it works is required. Ca(2+)-dependent and -independent phosphorylation and the direct binding of Ca(2+) to the essential light chain have been implicated in the regulation of MyoA activity. Here we identify a second essential light chain of MyoA and demonstrate the importance of both to Toxoplasma motility. We also investigate the role of Ca(2+) and the MyoA regulatory site in parasite motility and identify a potential mechanism whereby binding of a divalent cation to the essential light chains could stabilize the myosin to allow productive movement.
Copyright © 2015 Williams et al.
Figures



References
-
- Baum J, Richard D, Healer J, Rug M, Krnajski Z, Gilberger T-W, Green JL, Holder AA, Cowman AF. 2006. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem 281:5197–5208. doi:10.1074/jbc.M509807200. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
