Three-dimensional morphogenesis of MDCK cells induced by cellular contractile forces on a viscous substrate
- PMID: 26374384
- PMCID: PMC4571640
- DOI: 10.1038/srep14208
Three-dimensional morphogenesis of MDCK cells induced by cellular contractile forces on a viscous substrate
Abstract
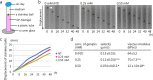
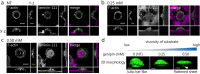
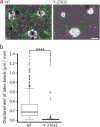
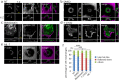
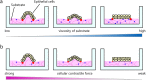
Substrate physical properties are essential for many physiological events such as embryonic development and 3D tissue formation. Physical properties of the extracellular matrix such as viscoelasticity and geometrical constraints are understood as factors that affect cell behaviour. In this study, we focused on the relationship between epithelial cell 3D morphogenesis and the substrate viscosity. We observed that Madin-Darby Canine Kidney (MDCK) cells formed 3D structures on a viscous substrate (Matrigel). The structures appear as a tulip hat. We then changed the substrate viscosity by genipin (GP) treatment. GP is a cross-linker of amino groups. Cells cultured on GP-treated-matrigel changed their 3D morphology in a substrate viscosity-dependent manner. Furthermore, to elucidate the spatial distribution of the cellular contractile force, localization of mono-phosphorylated and di-phosphorylated myosin regulatory light chain (P-MRLCs) was visualized by immunofluorescence. P-MRLCs localized along the periphery of epithelial sheets. Treatment with Y-27632, a Rho-kinase inhibitor, blocked the P-MRLCs localization at the edge of epithelial sheets and halted 3D morphogenesis. Our results indicate that the substrate viscosity, the substrate deformation, and the cellular contractile forces induced by P-MRLCs play crucial roles in 3D morphogenesis.
Figures

Similar articles
-
Pak1 regulates branching morphogenesis in 3D MDCK cell culture by a PIX and beta1-integrin-dependent mechanism.Am J Physiol Cell Physiol. 2010 Jul;299(1):C21-32. doi: 10.1152/ajpcell.00543.2009. Epub 2010 Mar 24. Am J Physiol Cell Physiol. 2010. PMID: 20457839 Free PMC article.
-
Epithelial morphogenesis of MDCK cells in three-dimensional collagen culture is modulated by interleukin-8.Am J Physiol Cell Physiol. 2013 May 15;304(10):C966-75. doi: 10.1152/ajpcell.00261.2012. Epub 2013 Mar 13. Am J Physiol Cell Physiol. 2013. PMID: 23485708 Free PMC article.
-
Effects of src kinase and TGFbeta1 on the differentiation and morphogenesis of MDCK cells grown in three-dimensional collagen and Matrigel environments.J Pathol. 2001 Oct;195(3):391-400. doi: 10.1002/path.949. J Pathol. 2001. PMID: 11673839
-
Differential sensitivity of epithelial cells to extracellular matrix in polarity establishment.PLoS One. 2014 Nov 13;9(11):e112922. doi: 10.1371/journal.pone.0112922. eCollection 2014. PLoS One. 2014. PMID: 25393292 Free PMC article.
-
Hydrostatic pressure as a driver of cell and tissue morphogenesis.Semin Cell Dev Biol. 2022 Nov;131:134-145. doi: 10.1016/j.semcdb.2022.04.021. Epub 2022 May 6. Semin Cell Dev Biol. 2022. PMID: 35534334 Free PMC article. Review.
Cited by
-
Giant peritoneal loose body and its protein composition: a case report.BMC Urol. 2024 Feb 17;24(1):43. doi: 10.1186/s12894-024-01425-8. BMC Urol. 2024. PMID: 38368330 Free PMC article.
-
Nanofiber-Mâché Hollow Ball Mimicking the Three-Dimensional Structure of a Cyst.Polymers (Basel). 2021 Jul 11;13(14):2273. doi: 10.3390/polym13142273. Polymers (Basel). 2021. PMID: 34301031 Free PMC article.
-
The cerebral cavernous malformation disease causing gene KRIT1 participates in intestinal epithelial barrier maintenance and regulation.FASEB J. 2019 Feb;33(2):2132-2143. doi: 10.1096/fj.201800343R. Epub 2018 Sep 25. FASEB J. 2019. PMID: 30252535 Free PMC article.
-
Spatial Fluctuations at Vertices of Epithelial Layers: Quantification of Regulation by Rho Pathway.Biophys J. 2018 Feb 27;114(4):939-946. doi: 10.1016/j.bpj.2017.12.026. Biophys J. 2018. PMID: 29490253 Free PMC article.
-
Cell-cell adhesion impacts epithelia response to substrate stiffness: Morphology and gene expression.Biophys J. 2022 Jan 18;121(2):336-346. doi: 10.1016/j.bpj.2021.11.2887. Epub 2021 Dec 2. Biophys J. 2022. PMID: 34864047 Free PMC article.
References
-
- O’Brien L. E., Zegers M. M. & Mostov K. E. Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol 3, 531–7 (2002). - PubMed
-
- Paszek M. J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–54 (2005). - PubMed
-
- Yeung T. et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60, 24–34 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

