Neoadjuvant 5-FU or Capecitabine Plus Radiation With or Without Oxaliplatin in Rectal Cancer Patients: A Phase III Randomized Clinical Trial
- PMID: 26374429
- PMCID: PMC4849360
- DOI: 10.1093/jnci/djv248
Neoadjuvant 5-FU or Capecitabine Plus Radiation With or Without Oxaliplatin in Rectal Cancer Patients: A Phase III Randomized Clinical Trial
Erratum in
-
Erratum.J Natl Cancer Inst. 2016 Mar 8;108(4):djw057. doi: 10.1093/jnci/djw057. Print 2016 Apr. J Natl Cancer Inst. 2016. PMID: 26957623 Free PMC article. No abstract available.
-
Corrigendum to "Neoadjuvant 5-FU or Capecitabine Plus Radiation With or Without Oxaliplatin in Rectal Cancer Patients: A Phase III Randomized Clinical Trial".J Natl Cancer Inst. 2018 Jul 1;110(7):794. doi: 10.1093/jnci/djy096. J Natl Cancer Inst. 2018. PMID: 29800481 Free PMC article. No abstract available.
Abstract
Background: National Surgical Adjuvant Breast and Bowel Project R-04 was designed to determine whether the oral fluoropyrimidine capecitabine could be substituted for continuous infusion 5-FU in the curative setting of stage II/III rectal cancer during neoadjuvant radiation therapy and whether the addition of oxaliplatin could further enhance the activity of fluoropyrimidine-sensitized radiation.
Methods: Patients with clinical stage II or III rectal cancer undergoing preoperative radiation were randomly assigned to one of four chemotherapy regimens in a 2x2 design: CVI 5-FU or oral capecitabine with or without oxaliplatin. The primary endpoint was local-regional tumor control. Time-to-event endpoint distributions were estimated using the Kaplan-Meier method. Hazard ratios were estimated from Cox proportional hazard models. All statistical tests were two-sided.
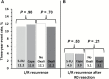
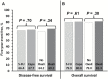
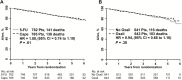
Results: Among 1608 randomized patients there were no statistically significant differences between regimens using 5-FU vs capecitabine in three-year local-regional tumor event rates (11.2% vs 11.8%), 5-year DFS (66.4% vs 67.7%), or 5-year OS (79.9% vs 80.8%); or for oxaliplatin vs no oxaliplatin for the three endpoints of local-regional events, DFS, and OS (11.2% vs 12.1%, 69.2% vs 64.2%, and 81.3% vs 79.0%). The addition of oxaliplatin was associated with statistically significantly more overall and grade 3-4 diarrhea (P < .0001). Three-year rates of local-regional recurrence among patients who underwent R0 resection ranged from 3.1 to 5.1% depending on the study arm.
Conclusions: Continuous infusion 5-FU produced outcomes for local-regional control, DFS, and OS similar to those obtained with oral capecitabine combined with radiation. This study establishes capecitabine as a standard of care in the pre-operative rectal setting. Oxaliplatin did not improve the local-regional failure rate, DFS, or OS for any patient risk group but did add considerable toxicity.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


Comment in
-
Curing more colorectal cancer.Natl Med J India. 2016 May-Jun;29(3):155-157. Natl Med J India. 2016. PMID: 27808066 No abstract available.
References
-
- Siegel R, Ma J, Zou Z, et al. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64 (5):9–29. - PubMed
-
- O’Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331 (8):502–507. - PubMed
-
- Van Cutsem E, Twelves C, Cassidy J, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19 (21):4097–4106. - PubMed
-
- Hoff PM, Ansari R, Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19 (8):2282–2292. - PubMed
-
- Glen H, Cassidy J. Redefining adjuvant chemotherapy in patients with stage III colon cancer: X-ACT trial. Expert Rev Anticancer Ther. 2008;8 (4):547–551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- U10-CA180868/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10-CA180821/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180822/CA/NCI NIH HHS/United States
- U10-CA180820/CA/NCI NIH HHS/United States
- UG1 CA189867/CA/NCI NIH HHS/United States
- UG1-CA189867/CA/NCI NIH HHS/United States
- U10-CA180888/CA/NCI NIH HHS/United States
- U10-CA180822/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

