Differential effects of leptin on adiponectin expression with weight gain versus obesity
- PMID: 26374448
- PMCID: PMC4747836
- DOI: 10.1038/ijo.2015.181
Differential effects of leptin on adiponectin expression with weight gain versus obesity
Abstract
Background/objective: Adiponectin exerts beneficial effects by reducing inflammation and improving lipid metabolism and insulin sensitivity. Although the adiponectin level is lower in obese individuals, whether weight gain reduces adiponectin expression in humans is controversial. We sought to investigate the role of weight gain, and consequent changes in leptin, on altering adiponectin expression in humans.
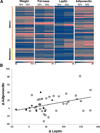
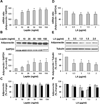
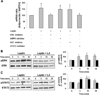
Methods/results: Forty-four normal-weight healthy subjects were recruited (mean age 29 years; 14 women) and randomized to either gain 5% of body weight by 8 weeks of overfeeding (n=34) or maintain weight (n=10). Modest weight gain of 3.8±1.2 kg resulted in increased adiponectin level (P=0.03), whereas weight maintenance resulted in no changes in adiponectin. Further, changes in adiponectin correlated positively with changes in leptin (P=0.0085). In-vitro experiments using differentiated human white preadipocytes showed that leptin increased adiponectin mRNA and protein expression, whereas a leptin antagonist had opposite effects. To understand the role of leptin in established obesity, we compared adipose tissue samples obtained from normal-weight versus obese subjects. We noted, first, that leptin activated cellular signaling pathways and increased adiponectin mRNA in the adipose tissue from normal-weight participants, but did not do so in the adipose tissue from obese participants. Second, we noted that obese subjects had increased caveolin-1 expression, which attenuates leptin-dependent increases in adiponectin.
Conclusions: Modest weight gain in healthy individuals is associated with increases in adiponectin levels, which correlate positively with changes in leptin. In vitro, leptin induces adiponectin expression, which is attenuated by increased caveolin-1 expression. In addition, the adipose tissue from obese subjects shows increased caveolin-1 expression and impaired leptin signaling. This leptin signal impairment may prevent concordant increases in adiponectin levels in obese subjects despite their high levels of leptin. Therefore, impaired leptin signaling may contribute to low adiponectin expression in obesity and may provide a target for increasing adiponectin expression, hence improving insulin sensitivity and cardio-metabolic profile in obesity.
Conflict of interest statement
Figures


Similar articles
-
Leptin signaling in adipose tissue: role in lipid accumulation and weight gain.Circ Res. 2012 Aug 17;111(5):599-603. doi: 10.1161/CIRCRESAHA.112.273656. Epub 2012 Jun 22. Circ Res. 2012. PMID: 22730441 Free PMC article.
-
Age and obesity-associated changes in the expression and activation of components of the AMPK signaling pathway in human right atrial tissue.Exp Gerontol. 2013 Jan;48(1):55-63. doi: 10.1016/j.exger.2012.04.005. Epub 2012 Apr 23. Exp Gerontol. 2013. PMID: 22546364
-
Adipose expression of adipocytokines in women with polycystic ovary syndrome.Fertil Steril. 2012 Jul;98(1):235-41. doi: 10.1016/j.fertnstert.2012.03.056. Epub 2012 May 16. Fertil Steril. 2012. PMID: 22607892
-
Recent advances in the relationship between obesity, inflammation, and insulin resistance.Eur Cytokine Netw. 2006 Mar;17(1):4-12. Eur Cytokine Netw. 2006. PMID: 16613757 Review.
-
Adipose tissue as an endocrine organ.Mol Cell Endocrinol. 2010 Mar 25;316(2):129-39. doi: 10.1016/j.mce.2009.08.018. Epub 2009 Aug 31. Mol Cell Endocrinol. 2010. PMID: 19723556 Review.
Cited by
-
Neuroendocrinological and Epigenetic Mechanisms Subserving Autonomic Imbalance and HPA Dysfunction in the Metabolic Syndrome.Front Neurosci. 2016 Apr 14;10:142. doi: 10.3389/fnins.2016.00142. eCollection 2016. Front Neurosci. 2016. PMID: 27147943 Free PMC article. Review.
-
Leptin/adiponectin ratio as a prognostic factor for increased weight gain in girls with central precocious puberty.Front Endocrinol (Lausanne). 2023 Mar 10;14:1101399. doi: 10.3389/fendo.2023.1101399. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36967781 Free PMC article.
-
Obesity: pathophysiology and therapeutic interventions.Mol Biomed. 2025 Apr 25;6(1):25. doi: 10.1186/s43556-025-00264-9. Mol Biomed. 2025. PMID: 40278960 Free PMC article. Review.
-
Periconceptional biomarkers for maternal obesity: a systematic review.Rev Endocr Metab Disord. 2023 Apr;24(2):139-175. doi: 10.1007/s11154-022-09762-5. Epub 2022 Dec 15. Rev Endocr Metab Disord. 2023. PMID: 36520252 Free PMC article.
-
Sarcopenic Obesity in Heart Failure With Preserved Ejection Fraction.Front Endocrinol (Lausanne). 2020 Sep 30;11:558271. doi: 10.3389/fendo.2020.558271. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33117276 Free PMC article. Review.
References
-
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. The Journal of biological chemistry. 1995;270(45):26746–26749. - PubMed
-
- Fruhbeck G. The Sir David Cuthbertson Medal Lecture. Hunting for new pieces to the complex puzzle of obesity. The Proceedings of the Nutrition Society. 2006;65(4):329–347. - PubMed
-
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. The Journal of biological chemistry. 1996;271(18):10697–10703. - PubMed
-
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochemical and biophysical research communications. 1999;257(1):79–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

