The myokine irisin increases cortical bone mass
- PMID: 26374841
- PMCID: PMC4593131
- DOI: 10.1073/pnas.1516622112
The myokine irisin increases cortical bone mass
Erratum in
-
Correction for Colaianni et al., The myokine irisin increases cortical bone mass.Proc Natl Acad Sci U S A. 2015 Oct 20;112(42):E5763. doi: 10.1073/pnas.1519137112. Epub 2015 Oct 1. Proc Natl Acad Sci U S A. 2015. PMID: 26430241 Free PMC article. No abstract available.
Abstract
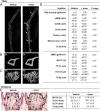
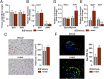
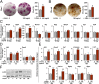
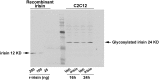
It is unclear how physical activity stimulates new bone synthesis. We explored whether irisin, a newly discovered myokine released upon physical activity, displays anabolic actions on the skeleton. Young male mice were injected with vehicle or recombinant irisin (r-irisin) at a low cumulative weekly dose of 100 µg kg(-1). We observed significant increases in cortical bone mass and strength, notably in cortical tissue mineral density, periosteal circumference, polar moment of inertia, and bending strength. This anabolic action was mediated primarily through the stimulation of bone formation, but with parallel notable reductions in osteoclast numbers. The trabecular compartment of the same bones was spared, as were vertebrae from the same mice. Higher irisin doses (3,500 µg kg(-1) per week) cause browning of adipose tissue; this was not seen with low-dose r-irisin. Expectedly, low-dose r-irisin modulated the skeletal genes, Opn and Sost, but not Ucp1 or Pparγ expression in white adipose tissue. In bone marrow stromal cell cultures, r-irisin rapidly phosphorylated Erk, and up-regulated Atf4, Runx2, Osx, Lrp5, β-catenin, Alp, and Col1a1; this is consistent with a direct receptor-mediated action to stimulate osteogenesis. We also noted that, although the irisin precursor Fndc5 was expressed abundantly in skeletal muscle, other sites, such as bone and brain, also expressed Fndc5, albeit at low levels. Furthermore, muscle fibers from r-irisin-injected mice displayed enhanced Fndc5 positivity, and irisin induced Fdnc5 mRNA expression in cultured myoblasts. Our data therefore highlight a previously unknown action of the myokine irisin, which may be the molecular entity responsible for muscle-bone connectivity.
Keywords: mechanical loading; osteoporosis; sarcopenia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Bone: Irisin boosts bone mass.Nat Rev Endocrinol. 2015 Dec;11(12):689. doi: 10.1038/nrendo.2015.174. Epub 2015 Oct 6. Nat Rev Endocrinol. 2015. PMID: 26437618 No abstract available.
References
-
- Dunstan D. Diabetes: Exercise and T2DM-move muscles more often! Nat Rev Endocrinol. 2011;7(4):189–190. - PubMed
-
- Baxter-Jones AD, Kontulainen SA, Faulkner RA, Bailey DA. A longitudinal study of the relationship of physical activity to bone mineral accrual from adolescence to young adulthood. Bone. 2008;43(6):1101–1107. - PubMed
-
- Andreoli A, Celi M, Volpe SL, Sorge R, Tarantino U. Long-term effect of exercise on bone mineral density and body composition in post-menopausal ex-elite athletes: A retrospective study. Eur J Clin Nutr. 2012;66(1):69–74. - PubMed
-
- Epstein S, Inzerillo AM, Caminis J, Zaidi M. Disorders associated with acute rapid and severe bone loss. J Bone Miner Res. 2003;18(12):2083–2094. - PubMed
-
- Keyak JH, Koyama AK, LeBlanc A, Lu Y, Lang TF. Reduction in proximal femoral strength due to long-duration spaceflight. Bone. 2009;44(3):449–453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

