The Triggering Receptor Expressed on Myeloid Cells 2 Binds Apolipoprotein E
- PMID: 26374897
- PMCID: PMC4646256
- DOI: 10.1074/jbc.M115.677286
The Triggering Receptor Expressed on Myeloid Cells 2 Binds Apolipoprotein E
Abstract
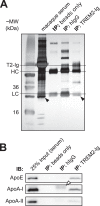
The triggering receptor expressed on myeloid cells 2 (TREM2) is an Ig-like V-type receptor expressed by populations of myeloid cells in the central nervous system and periphery. Loss-of-function mutations in TREM2 cause a progressive, fatal neurodegenerative disorder called Nasu-Hakola disease. In addition, a TREM2 R47H coding variant was recently identified as a risk factor for late-onset Alzheimer disease. TREM2 binds various polyanionic molecules but no specific protein ligands have been identified. Here we show that TREM2 specifically binds apolipoprotein E, a well established participant in Alzheimer disease. TREM2-Ig fusions efficiently precipitate ApoE from cerebrospinal fluid and serum. TREM2 also binds recombinant ApoE in solution and immobilized ApoE as detected by ELISA. Furthermore, the Alzheimer disease-associated R47H mutation, and other artificial mutations introduced in the same location, markedly reduced the affinity of TREM2 for ApoE. These findings reveal a link between two Alzheimer disease risk factors and may provide important clues to the pathogenesis of Nasu-Hakola disease and other neurodegenerative disorders.
Keywords: Alzheimer disease; Nasu-Hakola disease; PLOSL; TREM2; apolipoprotein E (ApoE); genetic polymorphism; high-density lipoprotein (HDL); myeloid cell; neurodegenerative disease.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Bouchon A., Dietrich J., and Colonna M. (2000) Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164, 4991–4995 - PubMed
-
- Daws M. R., Lanier L. L., Seaman W. E., and Ryan J. C. (2001) Cloning and characterization of a novel mouse myeloid DAP12-associated receptor family. Eur. J. Immunol. 31, 783–791 - PubMed
-
- Wunderlich P., Glebov K., Kemmerling N., Tien N. T., Neumann H., and Walter J. (2013) Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) protein by ectodomain shedding and γ-secretase-dependent intramembranous cleavage. J. Biol. Chem. 288, 33027–33036 - PMC - PubMed
-
- Zhong L., Chen X.-F., Zhang Z.-L., Wang Z., Shi X.-Z., Xu K., Zhang Y.-W., Xu H., and Bu G. (2015) DAP12 Stabilizes the C-terminal fragment of the triggering receptor expressed on myeloid cells-2 (TREM2) and protects against LPS-induced pro-inflammatory response. J. Biol. Chem. 290, 15866–15877 - PMC - PubMed
-
- Nasu T., Tsukahara Y., and Terayama K. (1973) A lipid metabolic disease-“membranous lipodystrophy”-an autopsy case demonstrating numerous peculiar membrane-structures composed of compound lipid in bone and bone marrow and various adipose tissues. Acta Pathol. Jpn. 23, 539–558 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

