RD3 loss dictates high-risk aggressive neuroblastoma and poor clinical outcomes
- PMID: 26375249
- PMCID: PMC4742193
- DOI: 10.18632/oncotarget.5204
RD3 loss dictates high-risk aggressive neuroblastoma and poor clinical outcomes
Abstract
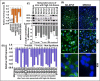
Clinical outcomes for high-risk neuroblastoma patients remains poor, with only 40-50% 5-Year overall survival (OS) and <10% long-term survival. The ongoing acquisition of genetic/molecular rearrangements in undifferentiated neural crest cells may endorse neuroblastoma progression. This study recognized the loss of Retinal Degeneration protein 3, RD3 in aggressive neuroblastoma, and identified its influence in better clinical outcomes and defined its novel metastasis suppressor function. The results showed ubiquitous expression of RD3 in healthy tissues, complete-loss and significant TNM-stage association of RD3 in clinical samples. RD3-loss was intrinsically associated with reduced OS, abridged relapse-free survival, aggressive stage etc., in neuroblastoma patient cohorts. RD3 was transcriptionally and translationally regulated in metastatic site-derived aggressive (MSDAC) cells (regardless of CSC status) ex vivo and in tumor manifolds from metastatic sites in reproducible aggressive disease models in vivo. Re-expressing RD3 in MSDACs reverted their metastatic potential both in vitro and in vivo. Conversely muting RD3 in neuroblastoma cells not only heightened invasion/migration but also dictated aggressive disease with metastasis. These results demonstrate the loss of RD3 in high-risk neuroblastoma, its novel, thus-far unrecognized metastasis suppressor function and further imply that RD3-loss may directly relate to tumor aggressiveness and poor clinical outcomes.
Keywords: RD3; high-risk aggressive neuroblastoma; metastasis; neuroblastoma; tumor suppressor.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Marc TG, Gurney JG, Smith MA, Olshan AF. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. National Cancer Institute; Bethesda, MD: 1999. Sympathetic Nervous System Tumors; pp. 65–72. NIH Pub. No. 99-4649(ICCC IV)
-
- Gurney JG, Smith MA, Ross JA. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. National Cancer Institute; Bethesda, MD: 1999. Cancer Among Infants; pp. 149–156. NIH Pub. No. 99–4649 (XII)
-
- Morgenstern DA, Baruchel S, Irwin MS. Current and future strategies for relapsed neuroblastoma: challenges on the road to precision therapy. Journal of pediatric hematology/oncology. 2013;35:337–347. - PubMed
-
- American-Cancer-Society . Cancer Facts & Figures. Atlanta: American Cancer Society; 2013. pp. 1–64.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

