Antigen-Specific Mammary Inflammation Depends on the Production of IL-17A and IFN-γ by Bovine CD4+ T Lymphocytes
- PMID: 26375594
- PMCID: PMC4573518
- DOI: 10.1371/journal.pone.0137755
Antigen-Specific Mammary Inflammation Depends on the Production of IL-17A and IFN-γ by Bovine CD4+ T Lymphocytes
Abstract
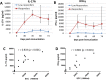
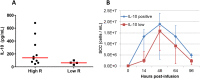
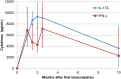
Intramammary infusion of the antigen used to sensitize cows by the systemic route induces a local inflammation associated with neutrophil recruitment. We hypothesize that this form of delayed type hypersensitivity, which may occur naturally during infections or could be induced intentionally by vaccination, can impact the outcome of mammary gland infections. We immunized cows with ovalbumin to identify immunological correlates of antigen-specific mammary inflammation. Intraluminal injection of ovalbumin induced a mastitis characterized by a prompt tissue reaction (increase in teat wall thickness) and an intense influx of leukocytes into milk of 10 responder cows out of 14 immunized animals. The magnitude of the local inflammatory reaction, assessed through milk leukocytosis, correlated with antibody titers, skin thickness test, and production of IL-17A and IFN-γ in a whole-blood antigen stimulation assay (WBA). The production of these two cytokines significantly correlated with the magnitude of the milk leukocytosis following the ovalbumin intramammary challenge. The IL-17A and IFN-γ production in the WBA was dependent on the presence of CD4+ cells in blood samples. In vitro stimulation of peripheral blood lymphocytes with ovalbumin followed by stimulation with PMA/ionomycin allowed the identification by flow cytometry of CD4+ T cells producing either IL-17A, IFN-γ, or both cytokines. The results indicate that the antigen-specific WBA, and specifically IL-17A and IFN-γ production by circulating CD4+ cells, can be used as a predictor of mammary hypersensitivity to protein antigens. This prompts further studies aiming at determining how Th17 and/or Th1 lymphocytes modulate the immune response of the mammary gland to infection.
Conflict of interest statement
Figures










Similar articles
-
Innate and Adaptive Immunity Synergize to Trigger Inflammation in the Mammary Gland.PLoS One. 2016 Apr 21;11(4):e0154172. doi: 10.1371/journal.pone.0154172. eCollection 2016. PLoS One. 2016. PMID: 27100324 Free PMC article.
-
T helper 17-associated cytokines are produced during antigen-specific inflammation in the mammary gland.PLoS One. 2013 May 16;8(5):e63471. doi: 10.1371/journal.pone.0063471. Print 2013. PLoS One. 2013. PMID: 23696826 Free PMC article.
-
IL-17A Is an Important Effector of the Immune Response of the Mammary Gland to Escherichia coli Infection.J Immunol. 2016 Jan 15;196(2):803-12. doi: 10.4049/jimmunol.1500705. Epub 2015 Dec 18. J Immunol. 2016. PMID: 26685206
-
Cells and cytokines in inflammatory secretions of bovine mammary gland.Adv Exp Med Biol. 2000;480:247-58. doi: 10.1007/0-306-46832-8_30. Adv Exp Med Biol. 2000. PMID: 10959433 Review.
-
Immune surveillance of mammary tissue by phagocytic cells.Adv Exp Med Biol. 2000;480:259-77. doi: 10.1007/0-306-46832-8_31. Adv Exp Med Biol. 2000. PMID: 10959434 Review.
Cited by
-
Adaptive Cell-Mediated Immunity in the Mammary Gland of Dairy Ruminants.Front Vet Sci. 2022 Apr 5;9:854890. doi: 10.3389/fvets.2022.854890. eCollection 2022. Front Vet Sci. 2022. PMID: 35464360 Free PMC article. Review.
-
Regulation of Inflammatory Responses of Cow Mammary Epithelial Cells through MAPK Signaling Pathways of IL-17A Cytokines.Animals (Basel). 2024 May 25;14(11):1572. doi: 10.3390/ani14111572. Animals (Basel). 2024. PMID: 38891619 Free PMC article.
-
Innate and Adaptive Immunity Synergize to Trigger Inflammation in the Mammary Gland.PLoS One. 2016 Apr 21;11(4):e0154172. doi: 10.1371/journal.pone.0154172. eCollection 2016. PLoS One. 2016. PMID: 27100324 Free PMC article.
-
Immunization of young heifers with staphylococcal immune evasion proteins before natural exposure to Staphylococcus aureus induces a humoral immune response in serum and milk.BMC Vet Res. 2019 Jan 7;15(1):15. doi: 10.1186/s12917-018-1765-9. BMC Vet Res. 2019. PMID: 30616609 Free PMC article.
-
Progress towards the Elusive Mastitis Vaccines.Vaccines (Basel). 2022 Feb 15;10(2):296. doi: 10.3390/vaccines10020296. Vaccines (Basel). 2022. PMID: 35214754 Free PMC article. Review.
References
-
- Halasa T, Huijps K, Osteras O, Hogeveen H. Economic effects of bovine mastitis and mastitis management: A review. Vet Q. 2007;29(1):18–31. - PubMed
-
- Bradley AJ. Bovine mastitis: An evolving disease. Vet J. 2002;164(2):116–28. - PubMed
-
- Craven N, Williams MR. Defences of the bovine mammary gland against infection and prospects for their enhancement. Vet Immunol Immunopathol. 1985;10(1):71–127. - PubMed
-
- Oviedo-Boyso J, Valdez-Alarcon JJ, Cajero-Juarez M, Ochoa-Zarzosa A, Lopez-Meza JE, Bravo-Patino A, et al. Innate immune response of bovine mammary gland to pathogenic bacteria responsible for mastitis. J Infect. 2007;54(4):399–409. - PubMed
-
- Rainard P, Riollet C. Innate immunity of the bovine mammary gland. Vet Res. 2006;37(3):369–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

