Helicobacter pylori neutrophil-activating protein induces release of histamine and interleukin-6 through G protein-mediated MAPKs and PI3K/Akt pathways in HMC-1 cells
- PMID: 26375619
- PMCID: PMC4826132
- DOI: 10.1080/21505594.2015.1043505
Helicobacter pylori neutrophil-activating protein induces release of histamine and interleukin-6 through G protein-mediated MAPKs and PI3K/Akt pathways in HMC-1 cells
Abstract
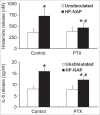
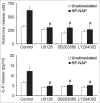
Helicobacter pylori neutrophil-activating protein (HP-NAP) activates several innate leukocytes including neutrophils, monocytes, and mast cells. It has been reported that HP-NAP induces degranulation and interleukin-6 (IL-6) secretion of rat peritoneal mast cells. However, the molecular mechanism is not very clear. Here, we show that HP-NAP activates human mast cell line-1 (HMC-1) cells to secrete histamine and IL-6. The secretion depends on pertussis toxin (PTX)-sensitive heterotrimeric G proteins but not on Toll-like receptor 2. Moreover, HP-NAP induces PTX-sensitive G protein-mediated activation of extracellular signal-regulated kinase 1/2 (ERK1/2), p38-mitogen-activated protein kinase (p38 MAPK), and Akt in HMC-1 cells. Inhibition of ERK1/2, p38 MAPK, or phosphatidylinositol 3-kinase (PI3K) suppresses HP-NAP-induced release of histamine and IL-6 from HMC-1 cells. Thus, the activation of HMC-1 cells by HP-NAP is through Gi-linked G protein-coupled receptor-mediated MAPKs and PI3K/Akt pathways.
Keywords: HP-NAP; Helicobacter pylori neutrophil-activating protein; PTX; TLR2; mast cells.
Figures




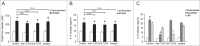




Similar articles
-
Helicobacter pylori Neutrophil-Activating Protein Directly Interacts with and Activates Toll-like Receptor 2 to Induce the Secretion of Interleukin-8 from Neutrophils and ATRA-Induced Differentiated HL-60 Cells.Int J Mol Sci. 2021 Oct 26;22(21):11560. doi: 10.3390/ijms222111560. Int J Mol Sci. 2021. PMID: 34768994 Free PMC article.
-
The neutrophil-activating protein of Helicobacter pylori (HP-NAP) activates the MAPK pathway in human neutrophils.Eur J Immunol. 2003 Apr;33(4):840-9. doi: 10.1002/eji.200323726. Eur J Immunol. 2003. PMID: 12672049
-
Helicobacter pylori heat-shock protein 60 induces interleukin-8 via a Toll-like receptor (TLR)2 and mitogen-activated protein (MAP) kinase pathway in human monocytes.J Med Microbiol. 2007 Feb;56(Pt 2):154-164. doi: 10.1099/jmm.0.46882-0. J Med Microbiol. 2007. PMID: 17244794
-
The neutrophil-activating protein of Helicobacter pylori (HP-NAP) as an immune modulating agent.FEMS Immunol Med Microbiol. 2007 Jul;50(2):157-64. doi: 10.1111/j.1574-695X.2007.00258.x. Epub 2007 May 22. FEMS Immunol Med Microbiol. 2007. PMID: 17521355 Review.
-
The Role of Helicobacter pylori Neutrophil-Activating Protein in the Pathogenesis of H. pylori and Beyond: From a Virulence Factor to Therapeutic Targets and Therapeutic Agents.Int J Mol Sci. 2022 Dec 21;24(1):91. doi: 10.3390/ijms24010091. Int J Mol Sci. 2022. PMID: 36613542 Free PMC article. Review.
Cited by
-
Role of Helicobacter pylori Eradication in Chronic Spontaneous Urticaria: A Propensity Score Matching Analysis.Clin Cosmet Investig Dermatol. 2021 Feb 5;14:129-136. doi: 10.2147/CCID.S293737. eCollection 2021. Clin Cosmet Investig Dermatol. 2021. PMID: 33584099 Free PMC article.
-
Abnormal transaminase and lipid profiles in coexisting diseases in patients with fatty liver: a population study in Sichuan.Biosci Rep. 2021 Dec 22;41(12):BSR20211769. doi: 10.1042/BSR20211769. Biosci Rep. 2021. PMID: 34918746 Free PMC article.
-
The Possible Role of Helicobacter pylori Infection in Non-alcoholic Fatty Liver Disease.Front Microbiol. 2017 May 10;8:743. doi: 10.3389/fmicb.2017.00743. eCollection 2017. Front Microbiol. 2017. PMID: 28539915 Free PMC article. Review.
-
Propofol attenuates mast cell degranulation via inhibiting the miR-221/PI3K/Akt/Ca2+ pathway.Exp Ther Med. 2018 Aug;16(2):1426-1432. doi: 10.3892/etm.2018.6317. Epub 2018 Jun 15. Exp Ther Med. 2018. PMID: 30116391 Free PMC article.
-
Candida albicans Sfp1 Is Involved in the Cell Wall and Endoplasmic Reticulum Stress Responses Induced by Human Antimicrobial Peptide LL-37.Int J Mol Sci. 2021 Sep 30;22(19):10633. doi: 10.3390/ijms221910633. Int J Mol Sci. 2021. PMID: 34638975 Free PMC article.
References
-
- Fu HW. Helicobacter pylori neutrophil-activating protein: from molecular pathogenesis to clinical applications. World J Gastroenterol 2014; 20:5294-301; PMID:24833859; http://dx.doi.org/ 10.3748/wjg.v20.i18.5294. - DOI - PMC - PubMed
-
- Wang CA, Liu YC, Du SY, Lin CW, Fu HW. Helicobacter pylori neutrophil-activating protein promotes myeloperoxidase release from human neutrophils. Biochem Biophys Res Commun 2008; 377:52-6; PMID:18823946; http://dx.doi.org/ 10.1016/j.bbrc.2008.09.072. - DOI - PubMed
-
- Polenghi A, Bossi F, Fischetti F, Durigutto P, Cabrelle A, Tamassia N, Cassatella MA, Montecucco C, Tedesco F, de Bernard M. The neutrophil-activating protein of Helicobacter pylori crosses endothelia to promote neutrophil adhesion in vivo. J Immunol 2007; 178:1312-20; http://dx.doi.org/ 10.4049/jimmunol.178.3.1312. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
