Role of bacteria in leukocyte adhesion deficiency-associated periodontitis
- PMID: 26375893
- PMCID: PMC4791199
- DOI: 10.1016/j.micpath.2015.09.003
Role of bacteria in leukocyte adhesion deficiency-associated periodontitis
Abstract
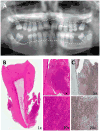
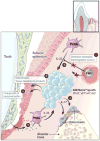
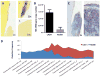
Leukocyte adhesion deficiency Type I (LAD-I)-associated periodontitis is an aggressive form of inflammatory bone loss that has been historically attributed to lack of neutrophil surveillance of the periodontal infection. However, this form of periodontitis has proven unresponsive to antibiotics and/or mechanical removal of the tooth-associated biofilm. Recent studies in LAD-I patients and relevant animal models have shown that the fundamental cause of LAD-I periodontitis involves dysregulation of a granulopoietic cytokine cascade. This cascade includes interleukin IL-23 (IL-23) and IL-17 that drive inflammatory bone loss in LAD-I patients and animal models and, moreover, foster a nutritionally favorable environment for bacterial growth and development of a compositionally unique microbiome. Although the lack of neutrophil surveillance in the periodontal pockets might be expected to lead to uncontrolled bacterial invasion of the underlying connective tissue, microbiological analyses of gingival biopsies from LAD-I patients did not reveal tissue-invasive infection. However, bacterial lipopolysaccharide was shown to translocate into the lesions of LAD-I periodontitis. It is concluded that the bacteria serve as initial triggers for local immunopathology through translocation of bacterial products into the underlying tissues where they unleash the dysregulated IL-23-IL-17 axis. Subsequently, the IL-23/IL-17 inflammatory response sustains and shapes a unique local microbiome which, in turn, can further exacerbate inflammation and bone loss in the susceptible host.
Keywords: IL-17; IL-23; Inflammation; Leukocyte adhesion deficiency; Microbiota; Periodontitis.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss.Sci Transl Med. 2014 Mar 26;6(229):229ra40. doi: 10.1126/scitranslmed.3007696. Sci Transl Med. 2014. PMID: 24670684 Free PMC article.
-
Subgingival microbial communities in Leukocyte Adhesion Deficiency and their relationship with local immunopathology.PLoS Pathog. 2015 Mar 5;11(3):e1004698. doi: 10.1371/journal.ppat.1004698. eCollection 2015 Mar. PLoS Pathog. 2015. PMID: 25741691 Free PMC article.
-
Etiology of leukocyte adhesion deficiency-associated periodontitis revisited: not a raging infection but a raging inflammatory response.Expert Rev Clin Immunol. 2014 Aug;10(8):973-5. doi: 10.1586/1744666X.2014.929944. Epub 2014 Jun 14. Expert Rev Clin Immunol. 2014. PMID: 24931458 Free PMC article. Review.
-
Frontline Science: Activation of metabolic nuclear receptors restores periodontal tissue homeostasis in mice with leukocyte adhesion deficiency-1.J Leukoc Biol. 2020 Nov;108(5):1501-1514. doi: 10.1002/JLB.5HI0420-648R. Epub 2020 May 18. J Leukoc Biol. 2020. PMID: 32421906 Free PMC article.
-
The presence, function and regulation of IL-17 and Th17 cells in periodontitis.J Clin Periodontol. 2014 Jun;41(6):541-9. doi: 10.1111/jcpe.12238. Epub 2014 Apr 15. J Clin Periodontol. 2014. PMID: 24735470 Review.
Cited by
-
Dysbiosis From a Microbial and Host Perspective Relative to Oral Health and Disease.Front Microbiol. 2021 Mar 5;12:617485. doi: 10.3389/fmicb.2021.617485. eCollection 2021. Front Microbiol. 2021. PMID: 33763040 Free PMC article. Review.
-
Longitudinal follow-up study of the association between statin use and chronic periodontitis using national health screening cohort of Korean population.Sci Rep. 2022 Apr 1;12(1):5504. doi: 10.1038/s41598-022-09540-y. Sci Rep. 2022. PMID: 35365732 Free PMC article.
-
Prevalence of two Entamoeba gingivalis ST1 and ST2-kamaktli subtypes in the human oral cavity under various conditions.Parasitol Res. 2018 Sep;117(9):2941-2948. doi: 10.1007/s00436-018-5990-8. Epub 2018 Jul 9. Parasitol Res. 2018. PMID: 29987412
-
Loss of Neutrophil Homing to the Periodontal Tissues Modulates the Composition and Disease Potential of the Oral Microbiota.Infect Immun. 2021 Nov 16;89(12):e0030921. doi: 10.1128/IAI.00309-21. Epub 2021 Sep 7. Infect Immun. 2021. PMID: 34491788 Free PMC article.
-
β2 Integrins-Multi-Functional Leukocyte Receptors in Health and Disease.Int J Mol Sci. 2020 Feb 19;21(4):1402. doi: 10.3390/ijms21041402. Int J Mol Sci. 2020. PMID: 32092981 Free PMC article. Review.
References
-
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–20. - PubMed
-
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. - PubMed
-
- Nussbaum G, Shapira L. How has neutrophil research improved our understanding of periodontal pathogenesis? J Clin Periodontol. 2011;38:49–59. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

