A current and historical perspective on disparities in US childhood pneumococcal conjugate vaccine adherence and in rates of invasive pneumococcal disease: Considerations for the routinely-recommended, pediatric PCV dosing schedule in the United States
- PMID: 26376039
- PMCID: PMC4962742
- DOI: 10.1080/21645515.2015.1069452
A current and historical perspective on disparities in US childhood pneumococcal conjugate vaccine adherence and in rates of invasive pneumococcal disease: Considerations for the routinely-recommended, pediatric PCV dosing schedule in the United States
Abstract
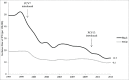
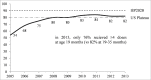
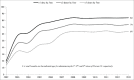
Previous research has suggested that reducing the US 4-dose PCV13 schedule to a 3-dose schedule may provide cost savings, despite more childhood pneumococcal disease. The study also stressed that dose reduction should be coupled with improved PCV adherence, however, US PCV uptake has leveled-off since 2008. An estimated 24-36% of US children aged 5-19 months are already receiving a reduced PCV schedule (i.e., missing ≥1 dose). This raises a practical concern that, under a reduced, 3-dose schedule, a similar proportion of children may receive ≤2 doses. It is also unknown if a reduced, 3-dose PCV schedule in the United States will afford the same disease protection as 3-dose schedules used elsewhere, given lower US PCV adherence. Finally, more assurance is needed that, under a reduced schedule, racial, socioeconomic, and geographic disparities in PCV adherence will not correspond with disproportionately higher rates of pneumococcal disease among poor or minority children.
Keywords: pneumococcal conjugate vaccine (PCV), adherence, coverage, dosing schedule, disparities, race, minorities, socioeconomic status, pneumococcal disease, 2+1, 3+1.
Figures
Similar articles
-
Adherence to pneumococcal conjugate vaccination schedule and uptake rate as compared to the established diphtheria-tetanus-acellular pertussis vaccination in Cyprus.Vaccine. 2018 Sep 11;36(38):5685-5691. doi: 10.1016/j.vaccine.2018.08.021. Epub 2018 Aug 14. Vaccine. 2018. PMID: 30115523
-
Pneumococcal vaccination in Europe: schedule adherence.Clin Ther. 2014 May;36(5):802-12.e1. doi: 10.1016/j.clinthera.2014.03.001. Epub 2014 Apr 18. Clin Ther. 2014. PMID: 24746990
-
Effectiveness of a 2+1 dose schedule pneumococcal conjugate vaccination programme on invasive pneumococcal disease among children in Norway.Vaccine. 2008 Jun 19;26(26):3277-81. doi: 10.1016/j.vaccine.2008.03.087. Epub 2008 Apr 18. Vaccine. 2008. PMID: 18456376
-
Introduction of pneumococcal conjugate vaccine into the public immunization program in South Africa: translating research into policy.Vaccine. 2012 Sep 7;30 Suppl 3:C21-7. doi: 10.1016/j.vaccine.2012.05.055. Vaccine. 2012. PMID: 22939016 Review.
-
Advances in pneumococcal disease prevention: 13-valent pneumococcal conjugate vaccine for infants and children.Clin Infect Dis. 2011 May;52(10):1241-7. doi: 10.1093/cid/cir142. Clin Infect Dis. 2011. PMID: 21507921 Review.
Cited by
-
Disparities in uptake of 13-valent pneumococcal conjugate vaccine among older adults in the United States.Hum Vaccin Immunother. 2019;15(4):841-849. doi: 10.1080/21645515.2018.1564434. Epub 2019 Feb 22. Hum Vaccin Immunother. 2019. PMID: 30676236 Free PMC article.
-
Herd immunity alters the conditions for performing dose schedule comparisons: an individual-based model of pneumococcal carriage.BMC Infect Dis. 2019 Mar 5;19(1):227. doi: 10.1186/s12879-019-3833-6. BMC Infect Dis. 2019. PMID: 30836941 Free PMC article.
-
Decision-making for PCV in adults.Hum Vaccin Immunother. 2019;15(3):584-593. doi: 10.1080/21645515.2018.1538611. Epub 2018 Dec 10. Hum Vaccin Immunother. 2019. PMID: 30352017 Free PMC article.
-
PCV13 Pediatric Routine Schedule Completion and Adherence Before and During the COVID-19 Pandemic in the United States.Infect Dis Ther. 2022 Dec;11(6):2141-2158. doi: 10.1007/s40121-022-00699-5. Epub 2022 Oct 10. Infect Dis Ther. 2022. PMID: 36219342 Free PMC article.
-
Disparities in invasive pneumococcal disease, pneumonia, and otitis media among US children by comorbidity profile and insurance status.Front Public Health. 2025 Jul 18;13:1558157. doi: 10.3389/fpubh.2025.1558157. eCollection 2025. Front Public Health. 2025. PMID: 40756406 Free PMC article.
References
-
- Advisory Committee on Immunization Practices, Preventing pneumococcal disease among infants and young children Recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep 2000; 49(RR-9):1-35 - PubMed
-
- Black SB, Shinefield HR, Hansen J, Elvin L, Laufer D, Malinoski F. Postlicensure evaluation of the effectiveness of seven valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2001; 20(12):1105-7; PMID:11740313; http://dx.doi.org/10.1097/00006454-200112000-00002 - DOI - PubMed
-
- Centers for Disease Control and Prevention . Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease–United States, 1998–2003. MMWR Morb Mortal Wkly Rep 2005; 54(36):893-7; PMID:16163262 - PubMed
-
- Kaplan SL, Mason EO Jr, Wald ER, Schutze GE, Bradley JS, Tan TQ, Hoffman JA, Givner LB, Yogev R, Barson WJ. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics 2004; 113(3 Pt 1):443-9; PMID:14993532; http://dx.doi.org/10.1542/peds.113.3.443 - DOI - PubMed
-
- Poehling KA, Talbot TR, Griffin MR, Craig AS, Whitney CG, Zell E, Lexau CA, Thomas AR, Harrison LH, Reingold AL. et al.. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA 2006; 295(14):1668-74; PMID:16609088; http://dx.doi.org/10.1001/jama.295.14.1668 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
