Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney disease and hyperkalemia
- PMID: 26376130
- PMCID: PMC4678168
- DOI: 10.1038/ki.2015.270
Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney disease and hyperkalemia
Abstract
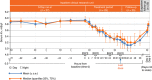
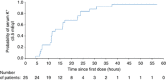
Patients with chronic kidney disease (CKD) have a high risk of hyperkalemia, which increases mortality and can lead to renin-angiotensin-aldosterone system inhibitor (RAASi) dose reduction or discontinuation. Patiromer, a nonabsorbed potassium binder, has been shown to normalize serum potassium in patients with CKD and hyperkalemia on RAASi. Here, patiromer's onset of action was determined in patients with CKD and hyperkalemia taking at least one RAASi. After a 3-day potassium- and sodium-restricted diet in an inpatient research unit, those with sustained hyperkalemia (serum potassium 5.5 - under 6.5 mEq/l) received patiromer 8.4 g/dose with morning and evening meals for a total of four doses. Serum potassium was assessed at baseline (0 h), 4 h postdose, then every 2-4 h to 48 h, at 58 h, and during outpatient follow-up. Mean baseline serum potassium was 5.93 mEq/l and was significantly reduced by 7 h after the first dose and at all subsequent times through 48 h. Significantly, mean serum potassium under 5.5 mEq/l was achieved within 20 h. At 48 h (14 h after last dose), there was a significant mean reduction of 0.75 mEq/l. Serum potassium did not increase before the next dose or for 24 h after the last dose. Patiromer was well tolerated, without serious adverse events and no withdrawals. The most common gastrointestinal adverse event was mild constipation in two patients. No hypokalemia (serum potassium under 3.5 mEq/l) was observed. Thus, patiromer induced an early and sustained reduction in serum potassium and was well tolerated in patients with CKD and sustained hyperkalemia on RAASis.
Figures


References
-
- 2Juurlink DN, Mamdani MM, Lee DS et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 2004; 351: 543–551. - PubMed
-
- 3Albert NM, Yancy CW, Liang L et al. Use of aldosterone antagonists in heart failure. JAMA 2009; 302: 1658–1665. - PubMed
-
- 4Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 2004; 351: 585–592. - PubMed
-
- 5Yildirim T, Arici M, Piskinpasa S et al. Major barriers against renin-angiotensin-aldosterone system blocker use in chronic kidney disease stages 3-5 in clinical practice: a safety concern? Renal Fail 2012; 34: 1095–1099. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

