New developments in the genetics, pathogenesis, and therapy of IgA nephropathy
- PMID: 26376134
- PMCID: PMC4653078
- DOI: 10.1038/ki.2015.252
New developments in the genetics, pathogenesis, and therapy of IgA nephropathy
Abstract
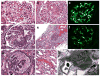
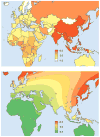
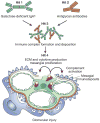
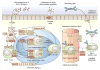
Recent years have brought notable progress in the field of IgA nephropathy. Here, we highlight important new directions and latest developments, including successful discovery of several genetic susceptibility loci, formulation of the multihit pathogenesis model, introduction of the Oxford pathology scoring system, and formalization of the Kidney Disease Improving Global Outcomes (KDIGO) consensus treatment guidelines. We focus on the latest genetic findings that confirm a strong contribution of inherited factors and explain some of the geoethnic disparities in disease susceptibility. Most IgA nephropathy susceptibility loci discovered to date encode genes involved in the maintenance of the intestinal epithelial barrier and response to mucosal pathogens. The concerted pattern of interpopulation allelic differentiation across all genetic loci parallels the disease prevalence and correlates with variation in local pathogens, suggesting that multilocus adaptation might have shaped the present-day landscape of IgA nephropathy. Importantly, the 'Intestinal Immune Network for IgA Production' emerged as one of the new targets for potential therapeutic intervention. We place these findings in the context of the multihit pathogenesis model and existing knowledge of IgA immunobiology. Lastly, we provide our perspective on the existing treatment options, discuss areas of clinical uncertainty, and outline ongoing clinical trials and translational studies.
Figures
References
-
- Berger J, Hinglais N. Intercapillary deposits of IgA-IgG. Journal d’urologie et de nephrologie. 1968;74:694–5. - PubMed
-
- Roberts IS. Pathology of IgA nephropathy. Nature reviews Nephrology. 2014;10:445–54. - PubMed
-
- Working Group of the International Ig ANN, the Renal Pathology S. Roberts IS, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney international. 2009;76:546–56. - PubMed
-
- Jennette JC. The immunohistology of IgA nephropathy. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1988;12:348–52. - PubMed
-
- Valentijn RM, Radl J, Haaijman JJ, et al. Circulating and mesangial secretory component-binding IgA-1 in primary IgA nephropathy. Kidney international. 1984;26:760–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

