Clinical Utility of a Plasma Protein Classifier for Indeterminate Lung Nodules
- PMID: 26376647
- PMCID: PMC4651976
- DOI: 10.1007/s00408-015-9800-0
Clinical Utility of a Plasma Protein Classifier for Indeterminate Lung Nodules
Abstract
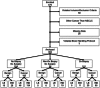
Evaluation of indeterminate pulmonary nodules is a complex challenge. Most are benign but frequently undergo invasive and costly procedures to rule out malignancy. A plasma protein classifier was developed that identifies likely benign nodules that can be triaged to CT surveillance to avoid unnecessary invasive procedures. The clinical utility of this classifier was assessed in a prospective-retrospective analysis of a study enrolling 475 patients with nodules 8-30 mm in diameter who had an invasive procedure to confirm diagnosis at 12 sites. Using this classifier, 32.0 % (CI 19.5-46.7) of surgeries and 31.8 % (CI 20.9-44.4) of invasive procedures (biopsy and/or surgery) on benign nodules could have been avoided. Patients with malignancy triaged to CT surveillance by the classifier would have been 24.0 % (CI 19.2-29.4). This rate is similar to that described in clinical practices (24.5 % CI 16.2-34.4). This study demonstrates the clinical utility of a non-invasive blood test for pulmonary nodules.
Trial registration: ClinicalTrials.gov NCT01752101.
Keywords: Biomarker; Clinical utility; Lung cancer; Lung nodule; Prospective; Xpresys lung.
Figures

References
-
- Vachani A, Tanner NT, Diette GB, Aggarwal J, Mathews C, Goss T, Kearney P, Fang K, Silvestri G. A Markov model derived to quantify drivers of decision making in the evaluation of patients with pulmonary nodules. Am J Repir Crit Care Med. 2014;189:A2259.
-
- Jensen T, Chin J, Ashby L. Hermansen J, Hutter JD (2015) Decision memo for screening for lung cancer with low dose computed tomography (LDCT). http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo..... Accessed 8 June 2015
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

