Heme Attenuation Ameliorates Irritant Gas Inhalation-Induced Acute Lung Injury
- PMID: 26376667
- PMCID: PMC4742996
- DOI: 10.1089/ars.2015.6347
Heme Attenuation Ameliorates Irritant Gas Inhalation-Induced Acute Lung Injury
Abstract
Aims: Exposure to irritant gases, such as bromine (Br2), poses an environmental and occupational hazard that results in severe lung and systemic injury. However, the mechanism(s) of Br2 toxicity and the therapeutic responses required to mitigate lung damage are not known. Previously, it was demonstrated that Br2 upregulates the heme degrading enzyme, heme oxygenase-1 (HO-1). Since heme is a major inducer of HO-1, we determined whether an increase in heme and heme-dependent oxidative injury underlies the pathogenesis of Br2 toxicity.
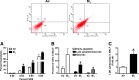
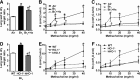
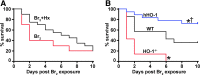
Results: C57BL/6 mice were exposed to Br2 gas (600 ppm, 30 min) and returned to room air. Thirty minutes postexposure, mice were injected intraperitoneally with a single dose of the heme scavenging protein, hemopexin (Hx) (3 μg/gm body weight), or saline. Twenty-four hours postexposure, saline-treated mice had elevated total heme in bronchoalveolar lavage fluid (BALF) and plasma and acute lung injury (ALI) culminating in 80% mortality after 10 days. Hx treatment significantly lowered heme, decreased evidence of ALI (lower protein and inflammatory cells in BALF, lower lung wet-to-dry weight ratios, and decreased airway hyperreactivity to methacholine), and reduced mortality. In addition, Br2 caused more severe ALI and mortality in mice with HO-1 gene deletion (HO-1-/-) compared to wild-type controls, while transgenic mice overexpressing the human HO-1 gene (hHO-1) showed significant protection.
Innovation: This is the first study delineating the role of heme in ALI caused by Br2.
Conclusion: The data suggest that attenuating heme may prove to be a useful adjuvant therapy to treat patients with ALI.
Figures






References
-
- Alam J. and Smith A. Heme-hemopexin-mediated induction of metallothionein gene expression. J Biol Chem 267: 16379–16384, 1992 - PubMed
-
- Alam J. and Smith A. Receptor-mediated transport of heme by hemopexin regulates gene expression in mammalian cells. J Biol Chem 264: 17637–17640, 1989 - PubMed
-
- Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, and Jordt SE. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L158–L172, 2014 - PMC - PubMed
-
- Bitron MD. and Aharonson EF. Delayed mortality of mice following inhalation of acute doses of CH2O, SO2Cl2, and Br2. Am Ind Hyg Assoc J 39: 129–138, 1978 - PubMed
-
- Brass CA, Immenschuh S, Song DX, Liem HH, and Eberhard UM. Hemopexin decreases spontaneous chemiluminescence of cold preserved liver after reperfusion. Biochem Biophys Res Commun 248: 574–577, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

