p16/Ki-67 Dual Stain Cytology for Detection of Cervical Precancer in HPV-Positive Women
- PMID: 26376685
- PMCID: PMC4675094
- DOI: 10.1093/jnci/djv257
p16/Ki-67 Dual Stain Cytology for Detection of Cervical Precancer in HPV-Positive Women
Abstract
Background: Human papillomavirus (HPV)-based cervical cancer screening requires triage markers to decide who should be referred to colposcopy. p16/Ki-67 dual stain cytology has been proposed as a biomarker for cervical precancers. We evaluated the dual stain in a large population of HPV-positive women.
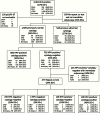
Methods: One thousand five hundred and nine HPV-positive women screened with HPV/cytology cotesting at Kaiser Permanente California were enrolled into a prospective observational study in 2012. Dual stain cytology was performed on residual Surepath material, and slides were evaluated for dual stain-positive cells. Disease endpoints were ascertained from the clinical database at KPNC. We evaluated the clinical performance of the assay among all HPV-positive women and among HPV-positive, cytology-negative women. We used internal benchmarks for clinical management to evaluate the clinical relevance of the dual stain assay. We evaluated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the dual stain compared with Pap cytology. All statistical tests were two-sided.
Results: The dual stain had lower positivity (45.9%) compared with cytology at an ASC-US threshold (53.4%). For detection of CIN2+, the dual stain had similar sensitivity (83.4% vs 76.6%, P = .1), and statistically higher specificity (58.9% vs 49.6%, P < .001), PPV (21.0% vs 16.6%, P < .001), and NPV (96.4% vs 94.2%, P = .01) compared with cytology. Similar patterns were observed for CIN3+. Women with a positive test had high enough risk for referral to colposcopy, while the risk for women with negative tests was below a one-year return threshold based on current US management guidelines.
Conclusion: Dual stain cytology showed good risk stratification for all HPV-positive women and for HPV-positive women with normal cytology. Additional follow-up is needed to determine how long dual stain negative women remain at low risk of precancer.
Published by Oxford University Press 2015. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

Comment in
-
RE: p16/Ki-67 Dual Stain Cytology for Detection of Cervical Precancer in HPV-Positive Women.J Natl Cancer Inst. 2015 Dec 27;108(2):djv389. doi: 10.1093/jnci/djv389. Print 2016 Feb. J Natl Cancer Inst. 2015. PMID: 26711872 No abstract available.
Similar articles
-
Five-Year Risk of Cervical Precancer Following p16/Ki-67 Dual-Stain Triage of HPV-Positive Women.JAMA Oncol. 2019 Feb 1;5(2):181-186. doi: 10.1001/jamaoncol.2018.4270. JAMA Oncol. 2019. PMID: 30325982 Free PMC article.
-
Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study.J Natl Cancer Inst. 2013 Oct 16;105(20):1550-7. doi: 10.1093/jnci/djt235. Epub 2013 Oct 4. J Natl Cancer Inst. 2013. PMID: 24096620 Free PMC article.
-
Performance of HPV Genotyping Combined with p16/Ki-67 in Detection of Cervical Precancer and Cancer Among HPV-Positive Chinese Women.Cancer Prev Res (Phila). 2020 Feb;13(2):163-172. doi: 10.1158/1940-6207.CAPR-19-0144. Epub 2019 Dec 23. Cancer Prev Res (Phila). 2020. PMID: 31871224
-
p16/ki-67 dual stain triage of individuals positive for HPV to detect cervical precancerous lesions.Int J Cancer. 2025 Jun 15;156(12):2257-2264. doi: 10.1002/ijc.35353. Epub 2025 Feb 4. Int J Cancer. 2025. PMID: 39901857 Free PMC article. Review.
-
p16INK4a/Ki67 Dual-Staining Immunocytochemistry to Refer Women Infected by High-Risk Human Papillomavirus for Colposcopy.Acta Cytol. 2025;69(1):16-25. doi: 10.1159/000542504. Epub 2025 Jan 17. Acta Cytol. 2025. PMID: 39827844 Review.
Cited by
-
The p16/ki-67 assay is a safe, effective and rapid approach to triage women with mild cervical lesions.PLoS One. 2021 Jun 11;16(6):e0253045. doi: 10.1371/journal.pone.0253045. eCollection 2021. PLoS One. 2021. PMID: 34115809 Free PMC article.
-
False positive cervical HPV screening test results.Papillomavirus Res. 2019 Jun;7:184-187. doi: 10.1016/j.pvr.2019.04.012. Epub 2019 Apr 25. Papillomavirus Res. 2019. PMID: 31029852 Free PMC article.
-
The effect of p16/Ki-67 and p16/mcm2 on the detection of cervical intraepithelial neoplasia: a prospective study from China.Int J Clin Exp Pathol. 2018 Aug 1;11(8):4101-4108. eCollection 2018. Int J Clin Exp Pathol. 2018. PMID: 31949801 Free PMC article.
-
Cost effectiveness analysis of HPV primary screening and dual stain cytology triage compared with cervical cytology.J Gynecol Oncol. 2019 Mar;30(2):e17. doi: 10.3802/jgo.2019.30.e17. Epub 2018 Nov 8. J Gynecol Oncol. 2019. PMID: 30740950 Free PMC article.
-
EpCAM expression in squamous cell carcinoma of the uterine cervix detected by monoclonal antibody to the membrane-proximal part of EpCAM.BMC Cancer. 2017 Dec 4;17(1):811. doi: 10.1186/s12885-017-3798-z. BMC Cancer. 2017. PMID: 29202724 Free PMC article.
References
-
- FDA approves first human papillomavirus test for primary cervical cancer screening. 2014. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm394773.htm
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

