Restoring Study 329: efficacy and harms of paroxetine and imipramine in treatment of major depression in adolescence
- PMID: 26376805
- PMCID: PMC4572084
- DOI: 10.1136/bmj.h4320
Restoring Study 329: efficacy and harms of paroxetine and imipramine in treatment of major depression in adolescence
Abstract
Objectives: To reanalyse SmithKline Beecham's Study 329 (published by Keller and colleagues in 2001), the primary objective of which was to compare the efficacy and safety of paroxetine and imipramine with placebo in the treatment of adolescents with unipolar major depression. The reanalysis under the restoring invisible and abandoned trials (RIAT) initiative was done to see whether access to and reanalysis of a full dataset from a randomised controlled trial would have clinically relevant implications for evidence based medicine.
Design: Double blind randomised placebo controlled trial.
Setting: 12 North American academic psychiatry centres, from 20 April 1994 to 15 February 1998.
Participants: 275 adolescents with major depression of at least eight weeks in duration. Exclusion criteria included a range of comorbid psychiatric and medical disorders and suicidality.
Interventions: Participants were randomised to eight weeks double blind treatment with paroxetine (20-40 mg), imipramine (200-300 mg), or placebo.
Main outcome measures: The prespecified primary efficacy variables were change from baseline to the end of the eight week acute treatment phase in total Hamilton depression scale (HAM-D) score and the proportion of responders (HAM-D score ≤8 or ≥50% reduction in baseline HAM-D) at acute endpoint. Prespecified secondary outcomes were changes from baseline to endpoint in depression items in K-SADS-L, clinical global impression, autonomous functioning checklist, self-perception profile, and sickness impact scale; predictors of response; and number of patients who relapse during the maintenance phase. Adverse experiences were to be compared primarily by using descriptive statistics. No coding dictionary was prespecified.
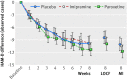
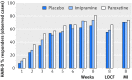
Results: The efficacy of paroxetine and imipramine was not statistically or clinically significantly different from placebo for any prespecified primary or secondary efficacy outcome. HAM-D scores decreased by 10.7 (least squares mean) (95% confidence interval 9.1 to 12.3), 9.0 (7.4 to 10.5), and 9.1 (7.5 to 10.7) points, respectively, for the paroxetine, imipramine and placebo groups (P=0.20). There were clinically significant increases in harms, including suicidal ideation and behaviour and other serious adverse events in the paroxetine group and cardiovascular problems in the imipramine group.
Conclusions: Neither paroxetine nor high dose imipramine showed efficacy for major depression in adolescents, and there was an increase in harms with both drugs. Access to primary data from trials has important implications for both clinical practice and research, including that published conclusions about efficacy and safety should not be read as authoritative. The reanalysis of Study 329 illustrates the necessity of making primary trial data and protocols available to increase the rigour of the evidence base.
© Le Noury et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures

Comment in
-
Restoring Study 329: results differ with the adverse event classification system used.BMJ. 2015 Oct 14;351:h5413. doi: 10.1136/bmj.h5413. BMJ. 2015. PMID: 26467710 No abstract available.
-
Authors' reply to Sasich and Linden.BMJ. 2015 Oct 14;351:h5412. doi: 10.1136/bmj.h5412. BMJ. 2015. PMID: 26467813 No abstract available.
-
Paroxetine and Study 329: what we already knew and when.BMJ. 2015 Oct 14;351:h5411. doi: 10.1136/bmj.h5411. BMJ. 2015. PMID: 26468042 No abstract available.
-
Both paroxetine and imipramine appear to be ineffective in adolescents with major depression, furthermore doubts have risen about their safety.Evid Based Med. 2016 Jun;21(3):92. doi: 10.1136/ebmed-2015-110320. Epub 2016 Feb 17. Evid Based Med. 2016. PMID: 26887424 No abstract available.
-
Reporting of clinical trials in nursing journals: how are we doing?J Adv Nurs. 2017 Dec;73(12):2782-2784. doi: 10.1111/jan.13149. Epub 2016 Sep 26. J Adv Nurs. 2017. PMID: 27627197 No abstract available.
References
-
- Keller MB, Ryan ND, Strober M, et al. Efficacy of paroxetine in the treatment of adolescent major depression: a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 2001;40:762-72. - PubMed
-
- McHenry L, Jureidini J. Industry-sponsored ghostwriting in clinical trial reporting: a case study. Account Res 2008;15:152-67. - PubMed
-
- Jureidini J, McHenry L, Mansfield P. Clinical trials and drug promotion: selective reporting of study 329. Int J Risk Saf Med 2008;20:73-81.
-
- Jureidini J, McHenry L. Conflicted medical journals and the failure of trust. Account Res 2011;18:45-54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
