The polarity protein Scrib mediates epidermal development and exerts a tumor suppressive function during skin carcinogenesis
- PMID: 26376988
- PMCID: PMC4574215
- DOI: 10.1186/s12943-015-0440-z
The polarity protein Scrib mediates epidermal development and exerts a tumor suppressive function during skin carcinogenesis
Abstract
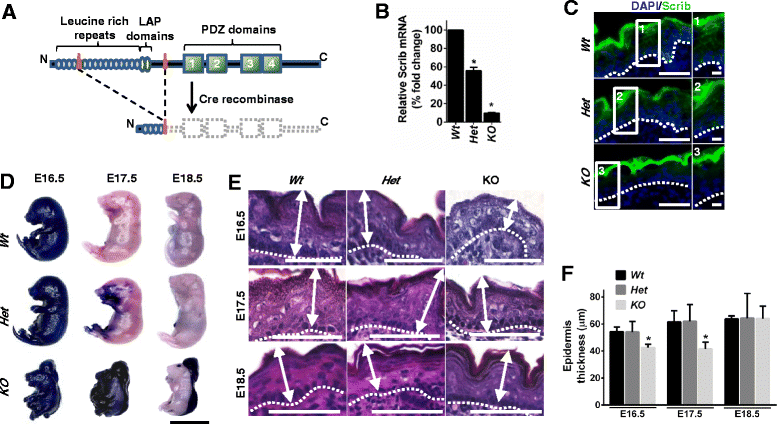
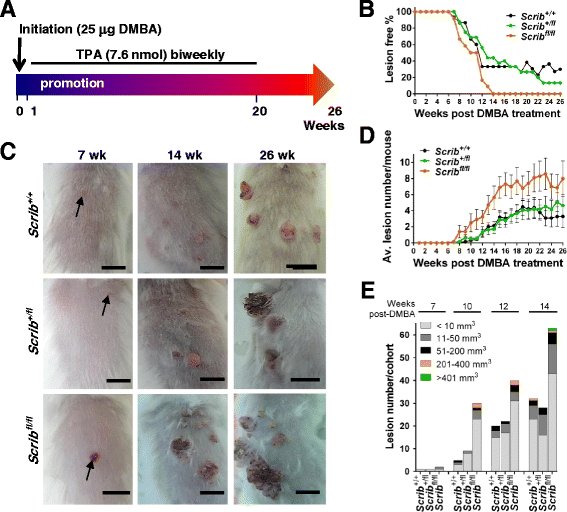
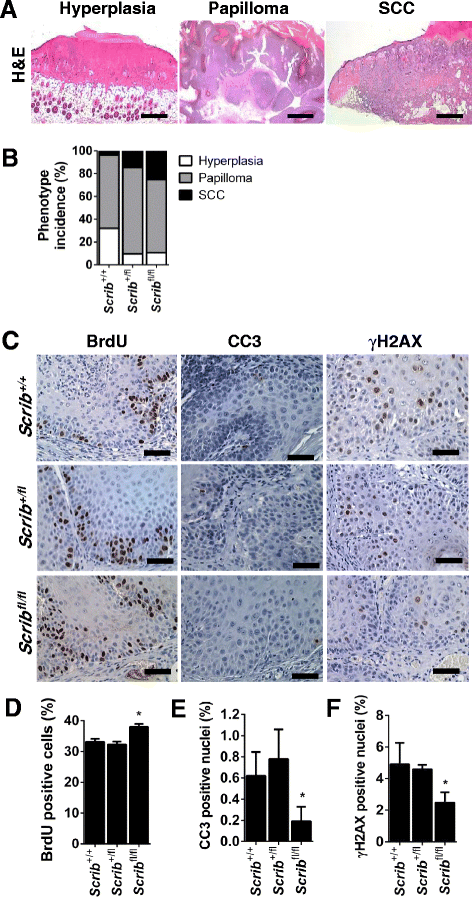
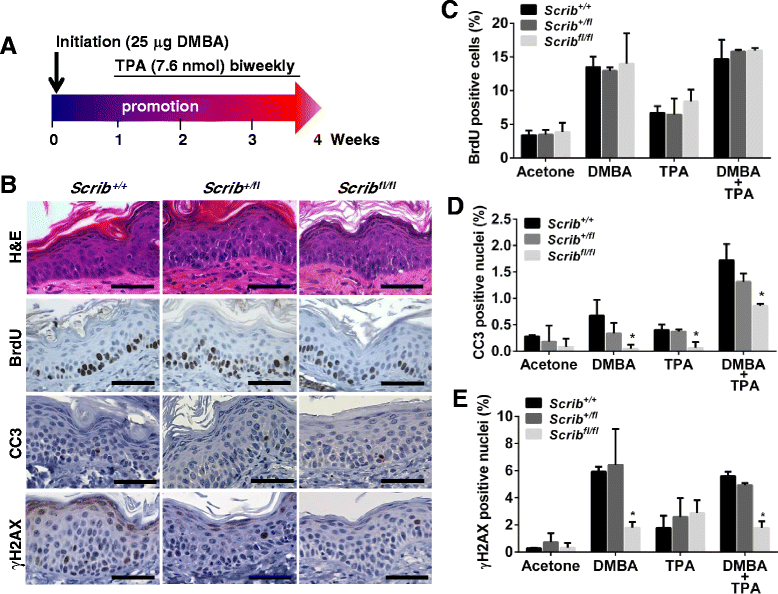
Background: The establishment and maintenance of polarity is vital for embryonic development and loss of polarity is a frequent characteristic of epithelial cancers, however the underlying molecular mechanisms remain unclear. Here, we identify a novel role for the polarity protein Scrib as a mediator of epidermal permeability barrier acquisition, skeletal morphogenesis, and as a potent tumor suppressor in cutaneous carcinogenesis.
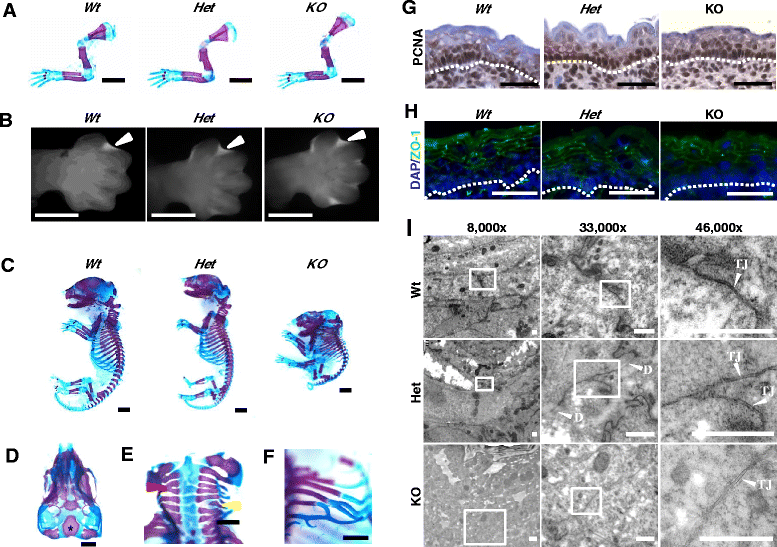
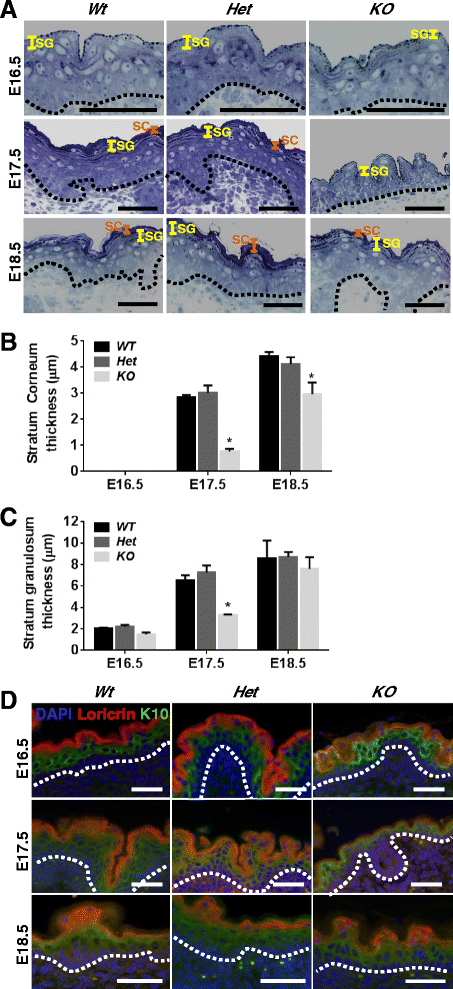
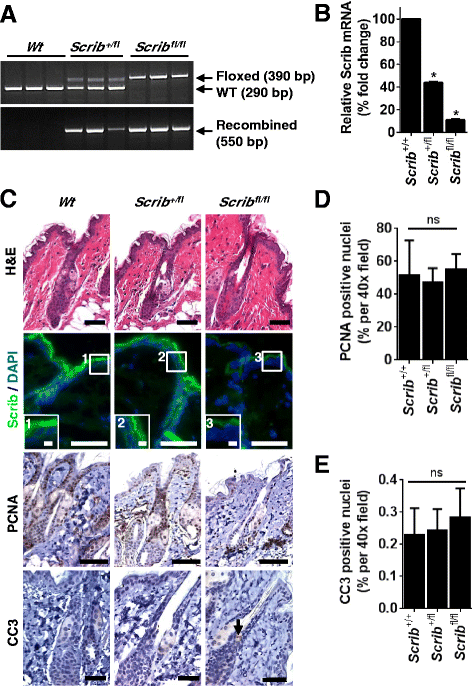
Methods: To explore the role of Scrib during epidermal development, we compared the permeability of toluidine blue dye in wild-type, Scrib heterozygous and Scrib KO embryonic epidermis at E16.5, E17.5 and E18.5. Mouse embryos were stained with alcian blue and alizarin red for skeletal analysis. To establish whether Scrib plays a tumor suppressive role during skin tumorigenesis and/or progression, we evaluated an autochthonous mouse model of skin carcinogenesis in the context of Scrib loss. We utilised Cre-LoxP technology to conditionally deplete Scrib in adult epidermis, since Scrib KO embryos are neonatal lethal.
Results: We establish that Scrib perturbs keratinocyte maturation during embryonic development, causing impaired epidermal barrier formation, and that Scrib is required for skeletal morphogenesis in mice. Analysis of conditional transgenic mice deficient for Scrib specifically within the epidermis revealed no skin pathologies, indicating that Scrib is dispensable for normal adult epidermal homeostasis. Nevertheless, bi-allelic loss of Scrib significantly enhanced tumor multiplicity and progression in an autochthonous model of epidermal carcinogenesis in vivo, demonstrating Scrib is an epidermal tumor suppressor. Mechanistically, we show that apoptosis is the critical effector of Scrib tumor suppressor activity during skin carcinogenesis and provide new insight into the function of polarity proteins during DNA damage repair.
Conclusions: For the first time, we provide genetic evidence of a unique link between skin carcinogenesis and loss of the epithelial polarity regulator Scrib, emphasizing that Scrib exerts a wide-spread tumor suppressive function in epithelia.
Figures
Similar articles
-
Deletion of 14-3-3σ sensitizes mice to DMBA/TPA-induced papillomatosis.Oncotarget. 2016 Jul 26;7(30):46862-46870. doi: 10.18632/oncotarget.10478. Oncotarget. 2016. PMID: 27409835 Free PMC article.
-
Loss of dynamin-related protein 1 (Drp1) does not affect epidermal development or UVB-induced apoptosis but does accelerate UVB-induced carcinogenesis.J Dermatol Sci. 2020 Aug;99(2):109-118. doi: 10.1016/j.jdermsci.2020.06.009. Epub 2020 Jun 26. J Dermatol Sci. 2020. PMID: 32636049
-
Genetic ablation of CCAAT/enhancer binding protein alpha in epidermis reveals its role in suppression of epithelial tumorigenesis.Cancer Res. 2007 Jul 15;67(14):6768-76. doi: 10.1158/0008-5472.CAN-07-0139. Cancer Res. 2007. PMID: 17638888 Free PMC article.
-
Epidermal polarity genes in health and disease.Cold Spring Harb Perspect Med. 2014 Dec 1;4(12):a015255. doi: 10.1101/cshperspect.a015255. Cold Spring Harb Perspect Med. 2014. PMID: 25452423 Free PMC article. Review.
-
Oncogene activation and tumor suppressor gene inactivation during multistage mouse skin carcinogenesis.Cancer Res. 1994 Apr 1;54(7 Suppl):1882s-1885s. Cancer Res. 1994. PMID: 8137304 Review.
Cited by
-
Scribble basal polarity acquisition in RPE cells and its mislocalization in a pathological AMD-like model.Front Neuroanat. 2022 Sep 23;16:983151. doi: 10.3389/fnana.2022.983151. eCollection 2022. Front Neuroanat. 2022. PMID: 36213611 Free PMC article.
-
Polarity in skin development and cancer.Curr Top Dev Biol. 2023;154:317-336. doi: 10.1016/bs.ctdb.2023.02.003. Epub 2023 Mar 10. Curr Top Dev Biol. 2023. PMID: 37100522 Free PMC article.
-
Scribble: A master scaffold in polarity, adhesion, synaptogenesis, and proliferation.J Cell Biol. 2019 Mar 4;218(3):742-756. doi: 10.1083/jcb.201810103. Epub 2018 Dec 31. J Cell Biol. 2019. PMID: 30598480 Free PMC article. Review.
-
Drosophila as a Model System to Study Nonautonomous Mechanisms Affecting Tumour Growth and Cell Death.Biomed Res Int. 2018 Mar 13;2018:7152962. doi: 10.1155/2018/7152962. eCollection 2018. Biomed Res Int. 2018. PMID: 29725601 Free PMC article. Review.
-
Lgl reduces endosomal vesicle acidification and Notch signaling by promoting the interaction between Vap33 and the V-ATPase complex.Sci Signal. 2018 Jun 5;11(533):eaar1976. doi: 10.1126/scisignal.aar1976. Sci Signal. 2018. PMID: 29871910 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

