SAGIT®: clinician-reported outcome instrument for managing acromegaly in clinical practice--development and results from a pilot study
- PMID: 26377024
- PMCID: PMC4710645
- DOI: 10.1007/s11102-015-0681-2
SAGIT®: clinician-reported outcome instrument for managing acromegaly in clinical practice--development and results from a pilot study
Abstract
Purpose: The SAGIT instrument is a comprehensive clinician-reported outcome instrument assessing key features of acromegaly: signs and symptoms, associated comorbidities; growth hormone levels; insulin-like growth factor-1 levels; and tumor profile. The SAGIT instrument has been designed to assist endocrinologists managing acromegaly in practice. Here, we report on pre-testing (to assess ease of understanding and acceptability) and a pilot study (to assess relevance, ease of use, and utility in real-life conditions) (NCT02231593).
Methods: For pre-testing, 11 endocrinologists completed the SAGIT instrument using patient medical records and were also interviewed. They subsequently completed a PRAgmatic Content and face validity Test (PRAC-Test(©)) to report their experiences using SAGIT, and feedback was used to revise the instrument. In the pilot study, nine endocrinologists completed the SAGIT instrument in real-time with patients belonging to three different categories (stable/controlled, active/uncontrolled acromegaly, treatment-naïve), while four completed the instrument based on medical-record review. All participants then completed the PRAC-Test(©) and their feedback was used to update the instrument.
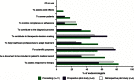
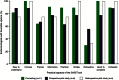
Results: The SAGIT instrument was well accepted by endocrinologists, with most indicating that it was concise, practical, easy to understand, useful for assessing treatment response, and valuable as a component of the patient's medical record. The pilot study confirmed the instrument's acceptability, utility, and ease of use, and indicated its potential for distinguishing acromegaly clinical stages.
Conclusions: The SAGIT instrument is promising as a tool for use by endocrinologists in everyday practice to assess the status and evolution of disease in patients with acromegaly and to guide treatment decision-making.
Keywords: Acromegaly; Clinician-reported outcomes; Instrument; Pilot study.
Figures



References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

