The effect of iron-fortified complementary food and intermittent preventive treatment of malaria on anaemia in 12- to 36-month-old children: a cluster-randomised controlled trial
- PMID: 26377199
- PMCID: PMC4573684
- DOI: 10.1186/s12936-015-0872-3
The effect of iron-fortified complementary food and intermittent preventive treatment of malaria on anaemia in 12- to 36-month-old children: a cluster-randomised controlled trial
Abstract
Background: Iron deficiency (ID) and malaria co-exist in tropical regions and both contribute to high rates of anaemia in young children. It is unclear whether iron fortification combined with intermittent preventive treatment (IPT) of malaria would be an efficacious strategy for reducing anaemia in young children.
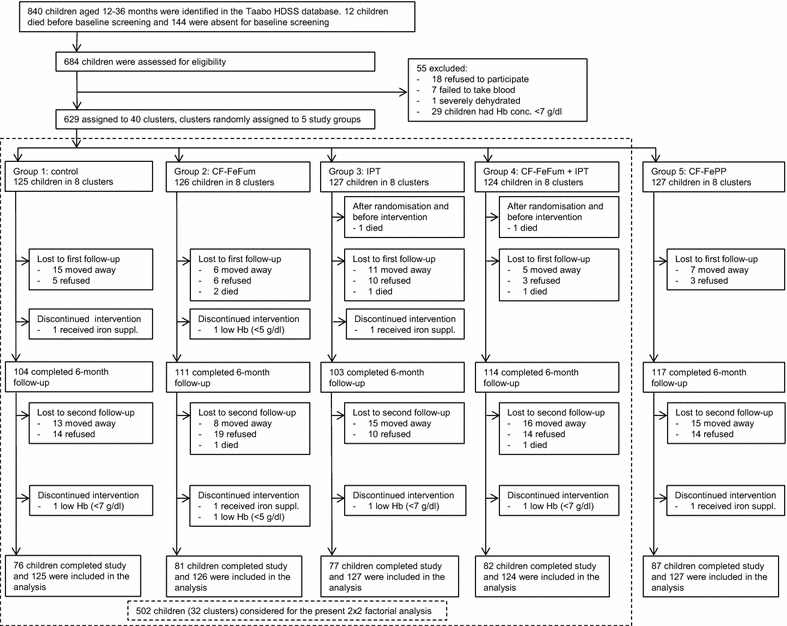
Methods: A 9-month cluster-randomised, single-blinded, placebo-controlled intervention trial was carried out in children aged 12-36 months in south-central Côte d'Ivoire, an area of intense and perennial malaria transmission. The study groups were: group 1: normal diet and IPT-placebo (n = 125); group 2: consumption of porridge, an iron-fortified complementary food (CF) with optimised composition providing 2 mg iron as NaFeEDTA and 3.8 mg iron as ferrous fumarate 6 days per week (CF-FeFum) and IPT-placebo (n = 126); group 3: IPT of malaria at 3-month intervals, using sulfadoxine-pyrimethamine and amodiaquine and no dietary intervention (n = 127); group 4: both CF-FeFum and IPT (n = 124); and group 5: consumption of porridge, an iron-fortified CF with the composition currently on the Ivorian market providing 2 mg iron as NaFeEDTA and 3.8 mg iron as ferric pyrophosphate 6 days per week (CF-FePP) and IPT-placebo (n = 127). The primary outcome was haemoglobin (Hb) concentration. Linear and logistic regression mixed-effect models were used for the comparison of the five study groups, and a 2 × 2 factorial analysis was used to assess treatment interactions of CF-FeFum and IPT (study groups 1-4).
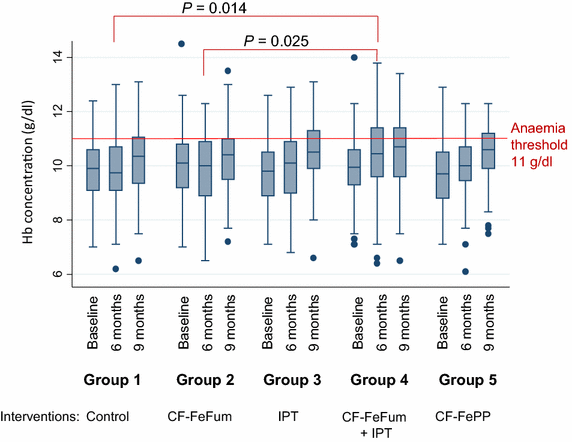
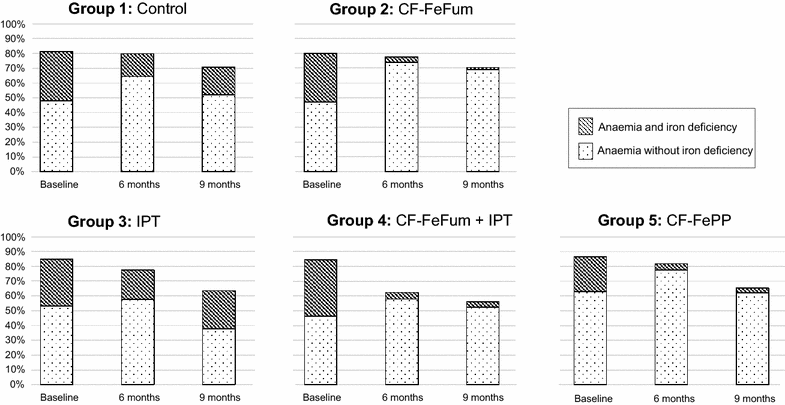
Results: After 9 months, the Hb concentration increased in all groups to a similar extent with no statistically significant difference between groups. In the 2 × 2 factorial analysis after 9 months, no treatment interaction was found on Hb (P = 0.89). The adjusted differences in Hb were 0.24 g/dl (95 % CI -0.10 to 0.59; P = 0.16) in children receiving IPT and -0.08 g/dl (95 % CI -0.42 to 0.26; P = 0.65) in children receiving CF-FeFum. At baseline, anaemia (Hb <11.0 g/dl) was 82.1 %. After 9 months, IPT decreased the odds of anaemia (odds ratio [OR], 0.46 [95 % CI 0.23-0.91]; P = 0.023), whereas iron-fortified CF did not (OR, 0.85 [95 % CI 0.43-1.68]; P = 0.68), although ID (plasma ferritin <30 μg/l) was decreased markedly in children receiving iron fortified CF (OR, 0.19 [95 % CI 0.09-0.40]; P < 0.001).
Conclusions: IPT alone only modestly decreased anaemia, but neither IPT nor iron fortified CF significantly improved Hb concentration after 9 months. Additionally, IPT did not augment the effect of the iron fortified CF. CF fortified with highly bioavailable iron improved iron status but not Hb concentration, despite three-monthly IPT of malaria. Thus, further research is necessary to develop effective combination strategies to prevent and treat anaemia in malaria endemic regions.
Trial registration: http://www.clinicaltrials.gov ; identifier NCT01634945; registered on July 3, 2012.
Figures
Similar articles
-
Iron Fortified Complementary Foods Containing a Mixture of Sodium Iron EDTA with Either Ferrous Fumarate or Ferric Pyrophosphate Reduce Iron Deficiency Anemia in 12- to 36-Month-Old Children in a Malaria Endemic Setting: A Secondary Analysis of a Cluster-Randomized Controlled Trial.Nutrients. 2017 Jul 14;9(7):759. doi: 10.3390/nu9070759. Nutrients. 2017. PMID: 28708072 Free PMC article. Clinical Trial.
-
Effect of intermittent preventive treatment of malaria on health and education in schoolchildren: a cluster-randomised, double-blind, placebo-controlled trial.Lancet. 2008 Jul 12;372(9633):127-138. doi: 10.1016/S0140-6736(08)61034-X. Lancet. 2008. PMID: 18620950 Free PMC article. Clinical Trial.
-
Intermittent administration of iron and sulfadoxine-pyrimethamine to control anaemia in Kenyan children: a randomised controlled trial.Lancet. 2002 Sep 21;360(9337):908-14. doi: 10.1016/S0140-6736(02)11027-0. Lancet. 2002. PMID: 12354473 Clinical Trial.
-
Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age (Review).Evid Based Child Health. 2013 Jan;8(1):112-201. doi: 10.1002/ebch.1895. Evid Based Child Health. 2013. PMID: 23878126 Review.
-
Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials.Lancet. 2009 Oct 31;374(9700):1533-42. doi: 10.1016/S0140-6736(09)61258-7. Epub 2009 Sep 16. Lancet. 2009. PMID: 19765816 Review.
Cited by
-
Intermittent preventive treatment for malaria in infants.Cochrane Database Syst Rev. 2021 Jul 17;7(7):CD011525. doi: 10.1002/14651858.CD011525.pub3. Cochrane Database Syst Rev. 2021. PMID: 34273901 Free PMC article.
-
High Awareness but Low Coverage of a Locally Produced Fortified Complementary Food in Abidjan, Côte d'Ivoire: Findings from a Cross-Sectional Survey.PLoS One. 2016 Nov 8;11(11):e0166295. doi: 10.1371/journal.pone.0166295. eCollection 2016. PLoS One. 2016. PMID: 27824917 Free PMC article.
-
How Eliminating Malaria May Also Prevent Iron Deficiency in African Children.Pharmaceuticals (Basel). 2018 Oct 1;11(4):96. doi: 10.3390/ph11040096. Pharmaceuticals (Basel). 2018. PMID: 30275421 Free PMC article. Review.
-
Intermittent preventive treatment for malaria in infants.Cochrane Database Syst Rev. 2019 Dec 2;12(12):CD011525. doi: 10.1002/14651858.CD011525.pub2. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Jul 17;7:CD011525. doi: 10.1002/14651858.CD011525.pub3. PMID: 31792925 Free PMC article. Updated.
-
Prevalence of Inherited Hemoglobin Disorders and Relationships with Anemia and Micronutrient Status among Children in Yaoundé and Douala, Cameroon.Nutrients. 2017 Jul 3;9(7):693. doi: 10.3390/nu9070693. Nutrients. 2017. PMID: 28671630 Free PMC article.
References
-
- Staubli Asobayire F, Adou P, Davidsson L, Cook JD, Hurrell RF. Prevalence of iron deficiency with and without concurrent anemia in population groups with high prevalences of malaria and other infections: a study in Côte d’Ivoire. Am J Clin Nutr. 2001;74:776–782. - PubMed
-
- Aponte JJ, Schellenberg D, Egan A, Breckenridge A, Carneiro I, Critchley J, et al. Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet. 2009;374:1533–1542. doi: 10.1016/S0140-6736(09)61258-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

