Characterization of Au and Bimetallic PtAu Nanoparticles on PDDA-Graphene Sheets as Electrocatalysts for Formic Acid Oxidation
- PMID: 26377218
- PMCID: PMC4573086
- DOI: 10.1186/s11671-015-1071-4
Characterization of Au and Bimetallic PtAu Nanoparticles on PDDA-Graphene Sheets as Electrocatalysts for Formic Acid Oxidation
Abstract
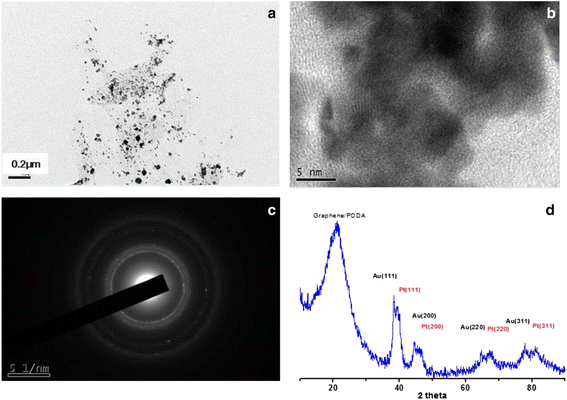
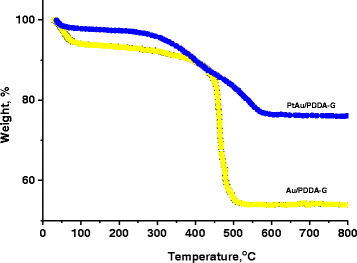
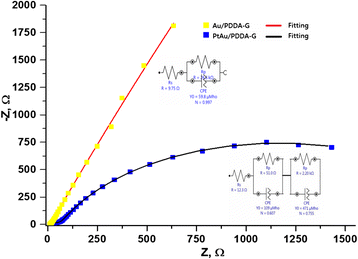
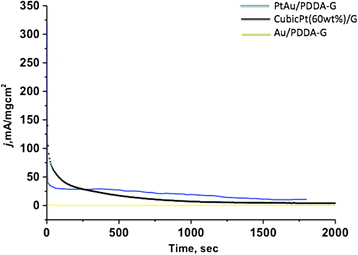
Nanocomposite materials of the Au nanoparticles (Au/PDDA-G) and the bimetallic PtAu nanoparticles on poly-(diallyldimethylammonium chloride) (PDDA)-modified graphene sheets (PtAu/PDDA-G) were prepared with hydrothermal method at 90 °C for 24 h. The composite materials Au/PDDA-G and PtAu/PDDA-G were evaluated by transmission electron microscopy (TEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and thermogravimetric analysis (TGA) for exploring the structural characterization for the electrochemical catalysis. According to TEM results, the diameter of Au and bimetallic PtAu nanoparticles is about 20-50 and 5-10 nm, respectively. X-ray diffraction (XRD) results indicate that both of PtAu and Au nanoparticles exhibit the crystalline plane of (111), (200), (210), and (311). Furthermore, XRD data also show the 2°-3° difference between pristine graphene sheets and the PDDA-modified graphene sheets. For the catalytic activity tests of Au/PDDA-G and PtAu/PDDA-G, the mixture of 0.5 M aqueous H2SO4 and 0.5 M aqueous formic acid was used as model to evaluate the electrochemical characterizations. The catalytic activities of the novel bimetallic PtAu/graphene electrocatalyst would be anticipated to be superior to the previous electrocatalyst of the cubic Pt/graphene.
Keywords: Au nanoparticle; Bimetallic PtAu nanoparticle; Formic acid oxidation; Graphene; PDDA.
Figures



References
-
- Yu X, Pickup PG. Recent advances in direct formic acid fuel cells (DAFAFC) J Power Sources. 2008;182:124–32. doi: 10.1016/j.jpowsour.2008.03.075. - DOI
-
- Ha S, Larsen R, Masel RI. Characterization and formic acid oxidation studies of PtAu nanoparticles. J Power Sources. 2005;144:28–34. doi: 10.1016/j.jpowsour.2004.12.031. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

