Chimeric Antigen Receptor T Cell Therapy in Hematology
- PMID: 26377367
- PMCID: PMC4805315
- DOI: 10.4274/tjh.2015.0049
Chimeric Antigen Receptor T Cell Therapy in Hematology
Abstract
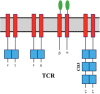
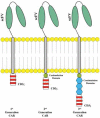
It is well demonstrated that the immune system can control and eliminate cancer cells. Immune-mediated elimination of tumor cells has been discovered and is the basis of both cancer vaccines and cellular therapies including hematopoietic stem cell transplantation. Adoptive T cell transfer has been improved to be more specific and potent and to cause less off-target toxicity. Currently, there are two forms of engineered T cells being tested in clinical trials: T cell receptor (TCR) and chimeric antigen receptor (CAR) modified T cells. On 1 July 2014, the United States Food and Drug Administration granted 'breakthrough therapy' designation to anti-CD19 CAR T cell therapy. Many studies were conducted to evaluate the benefits of this exciting and potent new treatment modality. This review summarizes the history of adoptive immunotherapy, adoptive immunotherapy using CARs, the CAR manufacturing process, preclinical and clinical studies, and the effectiveness and drawbacks of this strategy.
İmmün sistemin kanser hücrelerini kontrol ve elimine etme özelliğine sahip olduğu gösterilmiştir. İmmün-kontrollü eliminasyonda kanser aşıları ve hematopoietik kök hücre naklini içeren sellüler terapiler bulunmaktadır. Adoptif T hücre transferi daha potent ve spesifiktir, hedef dışı toksisitesi azdır. Klinik çalışmalarda iki tür T hücresi test edilmektedir: T hücre reseptör ve kimerik antijen reseptör (KAR) modifiye T hücreleri. 1 Temmuz 2014’te Amerikan Gıda ve İlaç Dairesi anti-CD19 ŞAR modifiye T hücre tedavisini “çığır açan tedaviler” sınıfına almıştır. Bu yeni tedavi yöntemini ve etkilerini araştıran birçok çalışma yapılmıştır. Bu derleme adoptif immünoterapinin geçmişini, ŞAR modifiye T hücrelerini, üretim sürecini, klinik ve preklinik çalışmaları özetlemektedir.
Conflict of interest statement
The authors of this paper have no conflicts of interest, including specific financial interests, relationships, and/or affiliations relevant to the subject matter or materials included.
Figures
Similar articles
-
RNA-transfection of γ/δ T cells with a chimeric antigen receptor or an α/β T-cell receptor: a safer alternative to genetically engineered α/β T cells for the immunotherapy of melanoma.BMC Cancer. 2017 Aug 17;17(1):551. doi: 10.1186/s12885-017-3539-3. BMC Cancer. 2017. PMID: 28818060 Free PMC article.
-
Engineered T cells: the promise and challenges of cancer immunotherapy.Nat Rev Cancer. 2016 Aug 23;16(9):566-81. doi: 10.1038/nrc.2016.97. Nat Rev Cancer. 2016. PMID: 27550819 Free PMC article. Review.
-
Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies.Immunol Rev. 2015 Jan;263(1):68-89. doi: 10.1111/imr.12243. Immunol Rev. 2015. PMID: 25510272 Review.
-
Genetically Modified T-Cell-Based Adoptive Immunotherapy in Hematological Malignancies.J Immunol Res. 2017;2017:5210459. doi: 10.1155/2017/5210459. Epub 2017 Jan 2. J Immunol Res. 2017. PMID: 28116322 Free PMC article. Review.
-
At The Bedside: Clinical review of chimeric antigen receptor (CAR) T cell therapy for B cell malignancies.J Leukoc Biol. 2016 Dec;100(6):1265-1272. doi: 10.1189/jlb.5BT1115-524R. Epub 2016 Jun 27. J Leukoc Biol. 2016. PMID: 27354412 Free PMC article. Review.
Cited by
-
Chimeric antigen T cell receptor treatment in hematological malignancies.Blood Res. 2019 Jun;54(2):81-83. doi: 10.5045/br.2019.54.2.81. Epub 2019 Jun 25. Blood Res. 2019. PMID: 31309082 Free PMC article. No abstract available.
-
Molecular properties of gp100-reactive T-cell receptors drive the cytokine profile and antitumor efficacy of transgenic host T cells.Pigment Cell Melanoma Res. 2019 Jan;32(1):68-78. doi: 10.1111/pcmr.12724. Epub 2018 Aug 13. Pigment Cell Melanoma Res. 2019. PMID: 30009548 Free PMC article.
-
Breakthroughs in modern cancer therapy and elusive cardiotoxicity: Critical research-practice gaps, challenges, and insights.Med Res Rev. 2018 Jan;38(1):325-376. doi: 10.1002/med.21463. Epub 2017 Sep 1. Med Res Rev. 2018. PMID: 28862319 Free PMC article. Review.
-
Novel CS1 CAR-T Cells and Bispecific CS1-BCMA CAR-T Cells Effectively Target Multiple Myeloma.Biomedicines. 2021 Oct 9;9(10):1422. doi: 10.3390/biomedicines9101422. Biomedicines. 2021. PMID: 34680541 Free PMC article.
-
Reducing Hinge Flexibility of CAR-T Cells Prolongs Survival In Vivo With Low Cytokines Release.Front Immunol. 2021 Oct 5;12:724211. doi: 10.3389/fimmu.2021.724211. eCollection 2021. Front Immunol. 2021. PMID: 34675920 Free PMC article.
References
-
- Sadelain M. CAR T cell therapy: the CD19 paradigm. Orlando, FL, USA: ASH Annual Meeting; 2014.
-
- Miller JS, Warren EH, Ritz J, Shlomchik WD, Murphy WJ, Barrett AJ, Kolb HJ, Giralt S, Bishop MR, Blazar BR, Falkenburg JH. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on the Biology Underlying Recurrence of Malignant Disease Following Allogeneic HSCT: Graft-versus-Tumor/Leukemia Reaction. Biol. Blood Marrow Transplant. 2010;16:565–586. - PMC - PubMed
-
- Ehrlich P. Über den jetzigen Stand der Karzinomforschung. Ned Tijdschr Geneeskd. 1909;5:273–290 (in German).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical