A mutual titer-enhancing relationship and similar localization patterns between Citrus exocortis viroid and Hop stunt viroid co-infecting two citrus cultivars
- PMID: 26377407
- PMCID: PMC4574207
- DOI: 10.1186/s12985-015-0357-6
A mutual titer-enhancing relationship and similar localization patterns between Citrus exocortis viroid and Hop stunt viroid co-infecting two citrus cultivars
Abstract
Background: Citrus exocortis viroid (CEVd) and Hop stunt viroid (HSVd) are commonly found simultaneously infecting different citrus cultivars in Taiwan. A crucial question to be addressed is how accumulations of these two viroids affect each other in an infected plant. In this study, we investigated the relationship between the two viroids at macroscopic and microscopic levels.
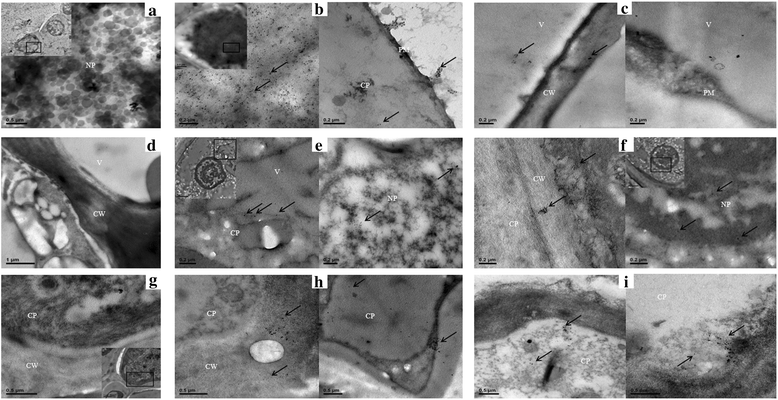
Methods: CEVd and HSVd titers were examined by real-time RT-PCR in 17 plants of two citrus cultivars (blood orange and Murcott mandarin) every 3 months (spring, summer, fall and winter) from 2011 to 2013. Three nonparametric tests (Spearman's rank correlation coefficient, Kendall's tau rank correlation coefficient and Hoeffding's inequality) were performed to test the correlation between CEVd and HSVd. Cellular and subcellular localizations of the two viroids were detected by digoxigenin- and colloidal gold-labeled in situ hybridization using light and transmission electron microscopy.
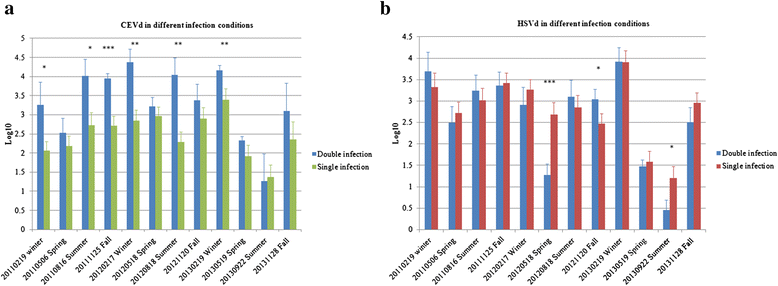
Results: The two viroids were unevenly distributed in four different types of citrus tissues (rootstock bark, roots, twig bark and leaves). Compared with blood orange, Murcott mandarin was generally more susceptible to CEVd and HSVd infection. Both viroids replicated and preferentially accumulated in the underground tissues of the two citrus cultivars. Except for blood orange at high temperatures, significant positive correlations were observed between the two viroids in specific tissues of both cultivars. Relative to concentrations under single-infection conditions, the CEVd population significantly increased under double infection during half of the 12 monitored seasons; in contrast, the population of HSVd significantly increased under double infection during only one season. At cellular/subcellular levels, the two viroids showed similar localization patterns in four tissues and the cells of these tissues in the two citrus cultivars.
Conclusions: Our findings of titer enhancement, localization similarity, and lack of symptom aggravation under CEVd and HSVd double infection suggest that the two viroids have a positive relationship in citrus. The combination of molecular and cellular techniques used in this study provided evidence of titer correlation and localization of co-infecting viroids in the host. These methods may thus be useful tools for exploring viroid-viroid and viroid-host interactions.
Figures



Similar articles
-
Multiplex detection, distribution, and genetic diversity of Hop stunt viroid and Citrus exocortis viroid infecting citrus in Taiwan.Virol J. 2015 Feb 3;12:11. doi: 10.1186/s12985-015-0247-y. Virol J. 2015. PMID: 25645458 Free PMC article.
-
Detection of Multiple Infections of Citrus exocortis viroid, Citrus viroid III, and Hop stunt viroid Variants in Hunan Province, China.Plant Dis. 2007 Sep;91(9):1205. doi: 10.1094/PDIS-91-9-1205A. Plant Dis. 2007. PMID: 30780682
-
Diagnostic real-time RT-PCR for the simultaneous detection of Citrus exocortis viroid and Hop stunt viroid.J Virol Methods. 2014 Feb;196:93-9. doi: 10.1016/j.jviromet.2013.11.001. Epub 2013 Nov 16. J Virol Methods. 2014. PMID: 24252553
-
A rapid isothermal assay for the detection of Hop stunt viroid in hop plants (Humulus lupulus), and its application in disease surveys.J Virol Methods. 2017 Jul;245:81-85. doi: 10.1016/j.jviromet.2017.04.002. Epub 2017 Apr 7. J Virol Methods. 2017. PMID: 28392409 Review.
-
The question of Citrus viroid IV as a cocadviroid.Arch Virol. 2005 Jun;150(6):1059-67. doi: 10.1007/s00705-005-0499-8. Epub 2005 Apr 13. Arch Virol. 2005. PMID: 15821974 Review.
Cited by
-
Evaluation of Disease Severity and Global Transcriptome Response Induced by Citrus bark cracking viroid, Hop latent viroid, and Their Co-Infection in Hop (Humulus lupulus L.).Int J Mol Sci. 2019 Jun 28;20(13):3154. doi: 10.3390/ijms20133154. Int J Mol Sci. 2019. PMID: 31261625 Free PMC article.
-
The Elimination of Viroids through In Vitro Thermotherapy and a Meristem Tip Culture from a New Limonime Hybrid (Citrus x limon var. limon (L.) Burm. f. x Citrus latifolia var. latifolia).BioTech (Basel). 2024 Sep 23;13(3):37. doi: 10.3390/biotech13030037. BioTech (Basel). 2024. PMID: 39329829 Free PMC article.
-
A Novel, Precise and High-Throughput Technology for Viroid Detection in Cannabis (MFDetectTM).Viruses. 2023 Jun 30;15(7):1487. doi: 10.3390/v15071487. Viruses. 2023. PMID: 37515174 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

