A scoping review of rapid review methods
- PMID: 26377409
- PMCID: PMC4574114
- DOI: 10.1186/s12916-015-0465-6
A scoping review of rapid review methods
Abstract
Background: Rapid reviews are a form of knowledge synthesis in which components of the systematic review process are simplified or omitted to produce information in a timely manner. Although numerous centers are conducting rapid reviews internationally, few studies have examined the methodological characteristics of rapid reviews. We aimed to examine articles, books, and reports that evaluated, compared, used or described rapid reviews or methods through a scoping review.
Methods: MEDLINE, EMBASE, the Cochrane Library, internet websites of rapid review producers, and reference lists were searched to identify articles for inclusion. Two reviewers independently screened literature search results and abstracted data from included studies. Descriptive analysis was conducted.
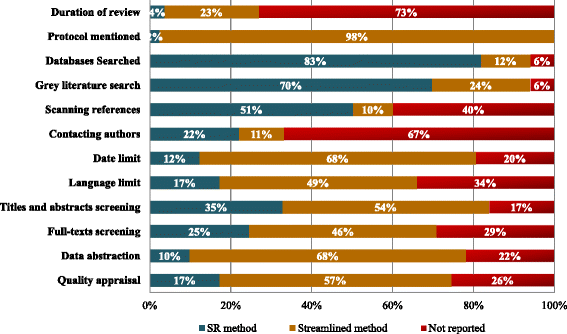
Results: We included 100 articles plus one companion report that were published between 1997 and 2013. The studies were categorized as 84 application papers, seven development papers, six impact papers, and four comparison papers (one was included in two categories). The rapid reviews were conducted between 1 and 12 months, predominantly in Europe (58 %) and North America (20 %). The included studies failed to report 6 % to 73 % of the specific systematic review steps examined. Fifty unique rapid review methods were identified; 16 methods occurred more than once. Streamlined methods that were used in the 82 rapid reviews included limiting the literature search to published literature (24 %) or one database (2 %), limiting inclusion criteria by date (68 %) or language (49 %), having one person screen and another verify or screen excluded studies (6 %), having one person abstract data and another verify (23 %), not conducting risk of bias/quality appraisal (7 %) or having only one reviewer conduct the quality appraisal (7 %), and presenting results as a narrative summary (78 %). Four case studies were identified that compared the results of rapid reviews to systematic reviews. Three studies found that the conclusions between rapid reviews and systematic reviews were congruent.
Conclusions: Numerous rapid review approaches were identified and few were used consistently in the literature. Poor quality of reporting was observed. A prospective study comparing the results from rapid reviews to those obtained through systematic reviews is warranted.
Figures



References
-
- Higgins JPT. Green S (Eds). Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (updated March 2011) Oxford: The Cochrane Collaboration; 2011.
-
- Petticrew M, Roberts H. Systematic reviews in the social sciences: a practical guide. Malden, MA: Blackwell Publishing Co.; 2006.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

