Individualised risk assessment for diabetic retinopathy and optimisation of screening intervals: a scientific approach to reducing healthcare costs
- PMID: 26377413
- PMCID: PMC4853547
- DOI: 10.1136/bjophthalmol-2015-307341
Individualised risk assessment for diabetic retinopathy and optimisation of screening intervals: a scientific approach to reducing healthcare costs
Abstract
Objective: To validate a mathematical algorithm that calculates risk of diabetic retinopathy progression in a diabetic population with UK staging (R0-3; M1) of diabetic retinopathy. To establish the utility of the algorithm to reduce screening frequency in this cohort, while maintaining safety standards.
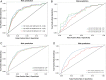
Research design and methods: The cohort of 9690 diabetic individuals in England, followed for 2 years. The algorithms calculated individual risk for development of preproliferative retinopathy (R2), active proliferative retinopathy (R3A) and diabetic maculopathy (M1) based on clinical data. Screening intervals were determined such that the increase in risk of developing certain stages of retinopathy between screenings was the same for all patients and identical to mean risk in fixed annual screening. Receiver operating characteristic curves were drawn and area under the curve calculated to estimate the prediction capability.
Results: The algorithm predicts the occurrence of the given diabetic retinopathy stages with area under the curve =80% for patients with type II diabetes (CI 0.78 to 0.81). Of the cohort 64% is at less than 5% risk of progression to R2, R3A or M1 within 2 years. By applying a 2 year ceiling to the screening interval, patients with type II diabetes are screened on average every 20 months, which is a 40% reduction in frequency compared with annual screening.
Conclusions: The algorithm reliably identifies patients at high risk of developing advanced stages of diabetic retinopathy, including preproliferative R2, active proliferative R3A and maculopathy M1. Majority of patients have less than 5% risk of progression between stages within a year and a small high-risk group is identified. Screening visit frequency and presumably costs in a diabetic retinopathy screening system can be reduced by 40% by using a 2 year ceiling. Individualised risk assessment with 2 year ceiling on screening intervals may be a pragmatic next step in diabetic retinopathy screening in UK, in that safety is maximised and cost reduced by about 40%.
Keywords: Clinical Trial; Diagnostic tests/Investigation; Epidemiology; Public health; Retina.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
