3D Shape Perception in Posterior Cortical Atrophy: A Visual Neuroscience Perspective
- PMID: 26377458
- PMCID: PMC4571603
- DOI: 10.1523/JNEUROSCI.3651-14.2015
3D Shape Perception in Posterior Cortical Atrophy: A Visual Neuroscience Perspective
Abstract
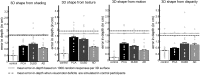
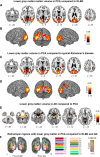
Posterior cortical atrophy (PCA) is a rare focal neurodegenerative syndrome characterized by progressive visuoperceptual and visuospatial deficits, most often due to atypical Alzheimer's disease (AD). We applied insights from basic visual neuroscience to analyze 3D shape perception in humans affected by PCA. Thirteen PCA patients and 30 matched healthy controls participated, together with two patient control groups with diffuse Lewy body dementia (DLBD) and an amnestic-dominant phenotype of AD, respectively. The hierarchical study design consisted of 3D shape processing for 4 cues (shading, motion, texture, and binocular disparity) with corresponding 2D and elementary feature extraction control conditions. PCA and DLBD exhibited severe 3D shape-processing deficits and AD to a lesser degree. In PCA, deficient 3D shape-from-shading was associated with volume loss in the right posterior inferior temporal cortex. This region coincided with a region of functional activation during 3D shape-from-shading in healthy controls. In PCA patients who performed the same fMRI paradigm, response amplitude during 3D shape-from-shading was reduced in this region. Gray matter volume in this region also correlated with 3D shape-from-shading in AD. 3D shape-from-disparity in PCA was associated with volume loss slightly more anteriorly in posterior inferior temporal cortex as well as in ventral premotor cortex. The findings in right posterior inferior temporal cortex and right premotor cortex are consistent with neurophysiologically based models of the functional anatomy of 3D shape processing. However, in DLBD, 3D shape deficits rely on mechanisms distinct from inferior temporal structural integrity.
Significance statement: Posterior cortical atrophy (PCA) is a neurodegenerative syndrome characterized by progressive visuoperceptual dysfunction and most often an atypical presentation of Alzheimer's disease (AD) affecting the ventral and dorsal visual streams rather than the medial temporal system. We applied insights from fundamental visual neuroscience to analyze 3D shape perception in PCA. 3D shape-processing deficits were affected beyond what could be accounted for by lower-order processing deficits. For shading and disparity, this was related to volume loss in regions previously implicated in 3D shape processing in the intact human and nonhuman primate brain. Typical amnestic-dominant AD patients also exhibited 3D shape deficits. Advanced visual neuroscience provides insight into the pathogenesis of PCA that also bears relevance for vision in typical AD.
Keywords: Alzheimer's disease; Lewy body; depth perception; fMRI; voxel-based morphometry.
Copyright © 2015 Gillebert, Schaeverbeke et al.
Figures









Similar articles
-
Clinical and radiological profile of posterior cortical atrophy and comparison with a group of typical Alzheimer disease and amnestic mild cognitive impairment.Acta Neurol Belg. 2021 Aug;121(4):1009-1018. doi: 10.1007/s13760-020-01547-4. Epub 2020 Nov 23. Acta Neurol Belg. 2021. PMID: 33230741
-
Functional Connectivity of Ventral and Dorsal Visual Streams in Posterior Cortical Atrophy.J Alzheimers Dis. 2016;51(4):1119-30. doi: 10.3233/JAD-150934. J Alzheimers Dis. 2016. PMID: 26923019
-
["I can't complete my payment form anymore" : Visual disorders in posterior cortical atrophy].Tijdschr Gerontol Geriatr. 2018 Sep;49(4):156-160. doi: 10.1007/s12439-018-0259-3. Epub 2018 Aug 6. Tijdschr Gerontol Geriatr. 2018. PMID: 30083812 Dutch.
-
Update on posterior cortical atrophy.Curr Opin Neurol. 2020 Feb;33(1):68-73. doi: 10.1097/WCO.0000000000000767. Curr Opin Neurol. 2020. PMID: 31688098 Free PMC article. Review.
-
Posterior cortical atrophy and Alzheimer's disease: a meta-analytic review of neuropsychological and brain morphometry studies.Brain Imaging Behav. 2013 Sep;7(3):353-61. doi: 10.1007/s11682-013-9236-1. Brain Imaging Behav. 2013. PMID: 23690254
Cited by
-
Convergence Along the Visual Hierarchy Is Altered in Posterior Cortical Atrophy.Invest Ophthalmol Vis Sci. 2020 Sep 1;61(11):8. doi: 10.1167/iovs.61.11.8. Invest Ophthalmol Vis Sci. 2020. PMID: 32897377 Free PMC article.
-
Detection and localisation of hesitant steps in people with Alzheimer's disease navigating routes of varying complexity.Healthc Technol Lett. 2019 Apr 24;6(2):42-47. doi: 10.1049/htl.2018.5034. eCollection 2019 Apr. Healthc Technol Lett. 2019. PMID: 31119037 Free PMC article.
-
The Dorsal Visual Pathway Represents Object-Centered Spatial Relations for Object Recognition.J Neurosci. 2022 Jun 8;42(23):4693-4710. doi: 10.1523/JNEUROSCI.2257-21.2022. Epub 2022 May 4. J Neurosci. 2022. PMID: 35508386 Free PMC article.
-
Extraction of three-dimensional shapes in glaucoma patients in response to monocular depth cues.Jpn J Ophthalmol. 2024 May;68(3):183-191. doi: 10.1007/s10384-024-01054-6. Epub 2024 Apr 10. Jpn J Ophthalmol. 2024. PMID: 38598144 Free PMC article.
-
Single-word comprehension deficits in the nonfluent variant of primary progressive aphasia.Alzheimers Res Ther. 2018 Jul 18;10(1):68. doi: 10.1186/s13195-018-0393-8. Alzheimers Res Ther. 2018. PMID: 30021613 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
