Calcitonin Gene-Related Peptide Reduces Taste-Evoked ATP Secretion from Mouse Taste Buds
- PMID: 26377461
- PMCID: PMC6795200
- DOI: 10.1523/JNEUROSCI.0100-15.2015
Calcitonin Gene-Related Peptide Reduces Taste-Evoked ATP Secretion from Mouse Taste Buds
Abstract
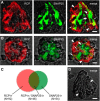
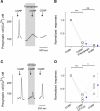
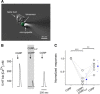
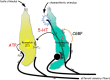
Immunoelectron microscopy revealed that peripheral afferent nerve fibers innervating taste buds contain calcitonin gene-related peptide (CGRP), which may be as an efferent transmitter released from peripheral axon terminals. In this report, we determined the targets of CGRP within taste buds and studied what effect CGRP exerts on taste bud function. We isolated mouse taste buds and taste cells, conducted functional imaging using Fura-2, and used cellular biosensors to monitor taste-evoked transmitter release. The findings showed that a subset of Presynaptic (Type III) taste cells (53%) responded to 0.1 μm CGRP with an increase in intracellular Ca(2+). In contrast, Receptor (Type II) taste cells rarely (4%) responded to 0.1 μm CGRP. Using pharmacological tools, the actions of CGRP were probed and elucidated by the CGRP receptor antagonist CGRP(8-37). We demonstrated that this effect of CGRP was dependent on phospholipase C activation and was prevented by the inhibitor U73122. Moreover, applying CGRP caused taste buds to secrete serotonin (5-HT), a Presynaptic (Type III) cell transmitter, but not ATP, a Receptor (Type II) cell transmitter. Further, our previous studies showed that 5-HT released from Presynaptic (Type III) cells provides negative paracrine feedback onto Receptor (Type II) cells by activating 5-HT1A receptors, and reducing ATP secretion. Our data showed that CGRP-evoked 5-HT release reduced taste-evoked ATP secretion. The findings are consistent with a role for CGRP as an inhibitory transmitter that shapes peripheral taste signals via serotonergic signaling during processing gustatory information in taste buds.
Significance statement: The taste sensation is initiated with a highly complex set of interactions between a variety of cells located within the taste buds before signal propagation to the brain. Afferent signals from the oral cavity are carried to the brain in chemosensory fibers that contribute to chemesthesis, the general chemical sensitivity of the mucus membranes in the oronasal cavities and being perceived as pungency, irritation, or heat. This is a study of a fundamental question in neurobiology: how are signals processed in sensory end organs, taste buds? More specifically, taste-modifying interactions, via transmitters, between gustatory and chemosensory afferents inside taste buds will help explain how a coherent output is formed before being transmitted to the brain.
Keywords: ATP; CGRP; Ca2+ imaging; biosensors; serotonin; taste.
Copyright © 2015 the authors 0270-6474/15/3512714-11$15.00/0.
Figures




Similar articles
-
Glutamate may be an efferent transmitter that elicits inhibition in mouse taste buds.PLoS One. 2012;7(1):e30662. doi: 10.1371/journal.pone.0030662. Epub 2012 Jan 26. PLoS One. 2012. PMID: 22292013 Free PMC article.
-
The effect of imiquimod on taste bud calcium transients and transmitter secretion.Br J Pharmacol. 2016 Nov;173(21):3121-3133. doi: 10.1111/bph.13567. Epub 2016 Sep 6. Br J Pharmacol. 2016. PMID: 27464850 Free PMC article.
-
Substance P as a putative efferent transmitter mediates GABAergic inhibition in mouse taste buds.Br J Pharmacol. 2018 Apr;175(7):1039-1053. doi: 10.1111/bph.14142. Epub 2018 Feb 23. Br J Pharmacol. 2018. PMID: 29328505 Free PMC article.
-
Taste buds as peripheral chemosensory processors.Semin Cell Dev Biol. 2013 Jan;24(1):71-9. doi: 10.1016/j.semcdb.2012.12.002. Epub 2012 Dec 20. Semin Cell Dev Biol. 2013. PMID: 23261954 Free PMC article. Review.
-
Cell communication in taste buds.Cell Mol Life Sci. 2006 Jul;63(13):1494-500. doi: 10.1007/s00018-006-6112-9. Cell Mol Life Sci. 2006. PMID: 16732426 Free PMC article. Review.
Cited by
-
Mouse Mandibular Retromolar Taste Buds Associated With a Mucus Salivary Gland.Chem Senses. 2021 Jan 1;46:bjab019. doi: 10.1093/chemse/bjab019. Chem Senses. 2021. PMID: 33855345 Free PMC article.
-
Altered gene expression in the lower respiratory tract of Car6 (-/-) mice.Transgenic Res. 2016 Oct;25(5):649-64. doi: 10.1007/s11248-016-9961-5. Epub 2016 May 21. Transgenic Res. 2016. PMID: 27209317
-
Pungency Perception and the Interaction with Basic Taste Sensations: An Overview.Foods. 2023 Jun 8;12(12):2317. doi: 10.3390/foods12122317. Foods. 2023. PMID: 37372528 Free PMC article. Review.
-
Taste buds: cells, signals and synapses.Nat Rev Neurosci. 2017 Aug;18(8):485-497. doi: 10.1038/nrn.2017.68. Epub 2017 Jun 29. Nat Rev Neurosci. 2017. PMID: 28655883 Free PMC article. Review.
-
Sensing Senses: Optical Biosensors to Study Gustation.Sensors (Basel). 2020 Mar 25;20(7):1811. doi: 10.3390/s20071811. Sensors (Basel). 2020. PMID: 32218129 Free PMC article. Review.
References
-
- Berg KA, Clarke WP, Sailstad C, Saltzman A, Maayani S. Signal transduction differences between 5-hydroxytryptamine type 2A and type 2C receptor systems. Mol Pharmacol. 1994;46:477–484. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
