Identified Serotonin-Releasing Neurons Induce Behavioral Quiescence and Suppress Mating in Drosophila
- PMID: 26377467
- PMCID: PMC6795202
- DOI: 10.1523/JNEUROSCI.1638-15.2015
Identified Serotonin-Releasing Neurons Induce Behavioral Quiescence and Suppress Mating in Drosophila
Abstract
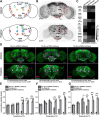
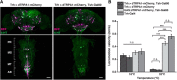
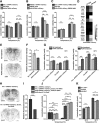
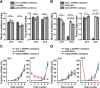
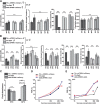
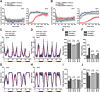
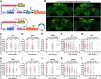
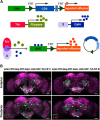
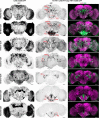
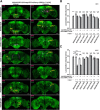
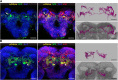
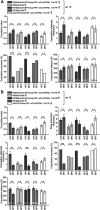
Animals show different levels of activity that are reflected in sensory responsiveness and endogenously generated behaviors. Biogenic amines have been determined to be causal factors for these states of arousal. It is well established that, in Drosophila, dopamine and octopamine promote increased arousal. However, little is known about factors that regulate arousal negatively and induce states of quiescence. Moreover, it remains unclear whether global, diffuse modulatory systems comprehensively affecting brain activity determine general states of arousal. Alternatively, individual aminergic neurons might selectively modulate the animals' activity in a distinct behavioral context. Here, we show that artificially activating large populations of serotonin-releasing neurons induces behavioral quiescence and inhibits feeding and mating. We systematically narrowed down a role of serotonin in inhibiting endogenously generated locomotor activity to neurons located in the posterior medial protocerebrum. We identified neurons of this cell cluster that suppress mating, but not feeding behavior. These results suggest that serotonin does not uniformly act as global, negative modulator of general arousal. Rather, distinct serotoninergic neurons can act as inhibitory modulators of specific behaviors.
Significance statement: An animal's responsiveness to external stimuli and its various types of endogenously generated, motivated behavior are highly dynamic and change between states of high activity and states of low activity. It remains unclear whether these states are mediated by unitary modulatory systems globally affecting brain activity, or whether distinct neurons modulate specific neuronal circuits underlying particular types of behavior. Using the model organism Drosophila melanogaster, we find that activating large proportions of serotonin-releasing neurons induces behavioral quiescence. Moreover, distinct serotonin-releasing neurons that we genetically isolated and identified negatively affect aspects of mating behavior, but not food uptake. This demonstrates that individual serotoninergic neurons can modulate distinct types of behavior selectively.
Keywords: Drosophila; arousal; motivational behavior; neuronal circuits; serotonin; thermogenetics.
Copyright © 2015 the authors 0270-6474/15/3512792-21$15.00/0.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
