Cardiac responses to hypoxia and reoxygenation in Drosophila
- PMID: 26377557
- PMCID: PMC4698404
- DOI: 10.1152/ajpregu.00164.2015
Cardiac responses to hypoxia and reoxygenation in Drosophila
Abstract
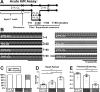
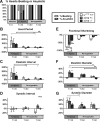
An adequate supply of oxygen is important for the survival of all tissues, but it is especially critical for tissues with high-energy demands, such as the heart. Insufficient tissue oxygenation occurs under a variety of conditions, including high altitude, embryonic and fetal development, inflammation, and thrombotic diseases, often affecting multiple organ systems. Responses and adaptations of the heart to hypoxia are of particular relevance in human cardiovascular and pulmonary diseases, in which the effects of hypoxic exposure can range in severity from transient to long-lasting. This study uses the genetic model system Drosophila to investigate cardiac responses to acute (30 min), sustained (18 h), and chronic (3 wk) hypoxia with reoxygenation. Whereas hearts from wild-type flies recovered quickly after acute hypoxia, exposure to sustained or chronic hypoxia significantly compromised heart function upon reoxygenation. Hearts from flies with mutations in sima, the Drosophila homolog of the hypoxia-inducible factor alpha subunit (HIF-α), exhibited exaggerated reductions in cardiac output in response to hypoxia. Heart function in hypoxia-selected flies, selected over many generations for survival in a low-oxygen environment, revealed reduced cardiac output in terms of decreased heart rate and fractional shortening compared with their normoxia controls. Hypoxia-selected flies also had smaller hearts, myofibrillar disorganization, and increased extracellular collagen deposition, consistent with the observed reductions in contractility. This study indicates that longer-duration hypoxic insults exert deleterious effects on heart function that are mediated, in part, by sima and advances Drosophila models for the genetic analysis of cardiac-specific responses to hypoxia and reoxygenation.
Keywords: Drosophila; genetic selection; heart; hypoxia; hypoxia-inducible factor; reoxygenation; sima.
Copyright © 2015 the American Physiological Society.
Figures





Comment in
-
Cardiac responses to hypoxia and reoxygenation in Drosophila. New insights into evolutionarily conserved gene responses. Focus on "Cardiac responses to hypoxia and reoxygenation in Drosophila".Am J Physiol Regul Integr Comp Physiol. 2015 Dec 1;309(11):R1344-6. doi: 10.1152/ajpregu.00419.2015. Epub 2015 Oct 7. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26447213 Free PMC article. No abstract available.
Similar articles
-
Cardiac responses to hypoxia and reoxygenation in Drosophila. New insights into evolutionarily conserved gene responses. Focus on "Cardiac responses to hypoxia and reoxygenation in Drosophila".Am J Physiol Regul Integr Comp Physiol. 2015 Dec 1;309(11):R1344-6. doi: 10.1152/ajpregu.00419.2015. Epub 2015 Oct 7. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26447213 Free PMC article. No abstract available.
-
Acute hypoxia induces sleep disorders via sima/HIF-1α regulation of circadian rhythms in adult Drosophila.Comp Biochem Physiol C Toxicol Pharmacol. 2025 Aug;294:110192. doi: 10.1016/j.cbpc.2025.110192. Epub 2025 Mar 12. Comp Biochem Physiol C Toxicol Pharmacol. 2025. PMID: 40086680
-
Cellular and developmental adaptations to hypoxia: a Drosophila perspective.Methods Enzymol. 2007;435:123-44. doi: 10.1016/S0076-6879(07)35007-6. Methods Enzymol. 2007. PMID: 17998052 Review.
-
The archipelago ubiquitin ligase subunit acts in target tissue to restrict tracheal terminal cell branching and hypoxic-induced gene expression.PLoS Genet. 2013;9(2):e1003314. doi: 10.1371/journal.pgen.1003314. Epub 2013 Feb 14. PLoS Genet. 2013. PMID: 23459416 Free PMC article.
-
Keeping the engine primed: HIF factors as key regulators of cardiac metabolism and angiogenesis during ischemia.J Mol Med (Berl). 2007 Dec;85(12):1309-15. doi: 10.1007/s00109-007-0279-x. Epub 2007 Nov 20. J Mol Med (Berl). 2007. PMID: 18026917 Review.
Cited by
-
Effects of Modest Hypoxia and Exercise on Cardiac Function, Sleep-Activity, Negative Geotaxis Behavior of Aged Female Drosophila.Front Physiol. 2020 Jan 21;10:1610. doi: 10.3389/fphys.2019.01610. eCollection 2019. Front Physiol. 2020. PMID: 32038290 Free PMC article.
-
What can the common fruit fly teach us about stroke?: lessons learned from the hypoxic tolerant Drosophila melanogaster.Front Cell Neurosci. 2024 Mar 22;18:1347980. doi: 10.3389/fncel.2024.1347980. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38584778 Free PMC article. Review.
-
Aging and the clock: Perspective from flies to humans.Eur J Neurosci. 2020 Jan;51(1):454-481. doi: 10.1111/ejn.14176. Epub 2018 Oct 30. Eur J Neurosci. 2020. PMID: 30269400 Free PMC article. Review.
-
Lifespan and ROS levels in different Drosophila melanogaster strains after 24 h hypoxia exposure.Biol Open. 2022 Jun 15;11(6):bio059386. doi: 10.1242/bio.059386. Epub 2022 Jun 29. Biol Open. 2022. PMID: 35616023 Free PMC article.
-
Cardiac responses to hypoxia and reoxygenation in Drosophila. New insights into evolutionarily conserved gene responses. Focus on "Cardiac responses to hypoxia and reoxygenation in Drosophila".Am J Physiol Regul Integr Comp Physiol. 2015 Dec 1;309(11):R1344-6. doi: 10.1152/ajpregu.00419.2015. Epub 2015 Oct 7. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26447213 Free PMC article. No abstract available.
References
-
- Azad P, Haddad GG. Survival in acute and severe low o environment: use of a genetic model system. Ann NY Acad Sci 1177: 39–47, 2009. - PubMed
-
- Bacon NC, Wappner P, O'Rourke JF, Bartlett SM, Shilo B, Pugh CW, Ratcliffe PJ. Regulation of the Drosophila bHLH-PAS protein Sima by hypoxia: functional evidence for homology with mammalian HIF-1 alpha. Biochem Biophys Res Commun 249: 811–816, 1998. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

