Brain endothelial dysfunction in cerebral adrenoleukodystrophy
- PMID: 26377633
- PMCID: PMC4731416
- DOI: 10.1093/brain/awv250
Brain endothelial dysfunction in cerebral adrenoleukodystrophy
Abstract
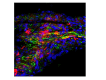
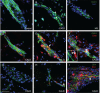
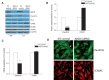
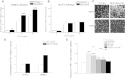
See Aubourg (doi:10.1093/awv271) for a scientific commentary on this article.X-linked adrenoleukodystrophy is caused by mutations in the ABCD1 gene leading to accumulation of very long chain fatty acids. Its most severe neurological manifestation is cerebral adrenoleukodystrophy. Here we demonstrate that progressive inflammatory demyelination in cerebral adrenoleukodystrophy coincides with blood-brain barrier dysfunction, increased MMP9 expression, and changes in endothelial tight junction proteins as well as adhesion molecules. ABCD1, but not its closest homologue ABCD2, is highly expressed in human brain microvascular endothelial cells, far exceeding its expression in the systemic vasculature. Silencing of ABCD1 in human brain microvascular endothelial cells causes accumulation of very long chain fatty acids, but much later than the immediate upregulation of adhesion molecules and decrease in tight junction proteins. This results in greater adhesion and transmigration of monocytes across the endothelium. PCR-array screening of human brain microvascular endothelial cells after ABCD1 silencing revealed downregulation of both mRNA and protein levels of the transcription factor c-MYC (encoded by MYC). Interestingly, MYC silencing mimicked the effects of ABCD1 silencing on CLDN5 and ICAM1 without decreasing the levels of ABCD1 protein itself. Together, these data demonstrate that ABCD1 deficiency induces significant alterations in brain endothelium via c-MYC and may thereby contribute to the increased trafficking of leucocytes across the blood-brain barrier as seen in cerebral adrenouleukodystrophy.
Keywords: blood–brain barrier; demyelination; genetics; leukodystrophy; neurodegeneration; neuroinflammation.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures




Comment in
-
Cerebral adrenoleukodystrophy: a demyelinating disease that leaves the door wide open.Brain. 2015 Nov;138(Pt 11):3133-6. doi: 10.1093/brain/awv271. Brain. 2015. PMID: 26503938 No abstract available.
References
-
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 2011; 14: 1142–9. - PubMed
-
- Bastaki M, Nelli EE, Dell'Era P, Rusnati M, Molinari-Tosatti MP, Parolini S, et al. Basic fibroblast growth factor-induced angiogenic phenotype in mouse endothelium. A study of aortic and microvascular endothelial cell lines. Arterioscler Thromb Vasc Biol 1997; 17: 454–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

