Type 2 immunity-dependent reduction of segmented filamentous bacteria in mice infected with the helminthic parasite Nippostrongylus brasiliensis
- PMID: 26377648
- PMCID: PMC4574229
- DOI: 10.1186/s40168-015-0103-8
Type 2 immunity-dependent reduction of segmented filamentous bacteria in mice infected with the helminthic parasite Nippostrongylus brasiliensis
Erratum in
-
Erratum to: Type 2 immunity-dependent reduction of segmented filamentous bacteria in mice infected with the helminthic parasite Nippostrongylus brasiliensis.Microbiome. 2015 Dec 21;3:77. doi: 10.1186/s40168-015-0142-1. Microbiome. 2015. PMID: 26689820 Free PMC article. No abstract available.
Abstract
Background: Dynamic interactions between the host and gastrointestinal microbiota play an important role for local and systemic immune homeostasis. Helminthic parasites modulate the host immune response, resulting in protection against autoimmune disease but also increased susceptibility to pathogen infection. The underlying mechanisms remain largely unknown.
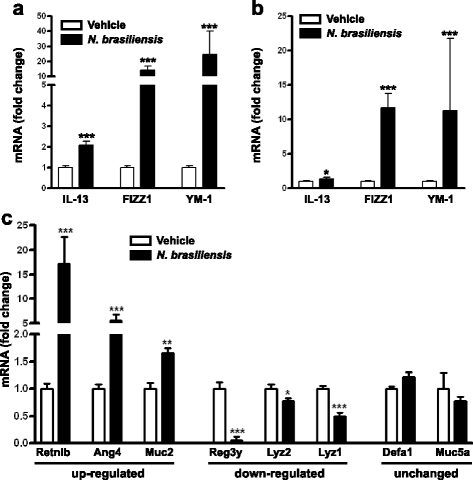
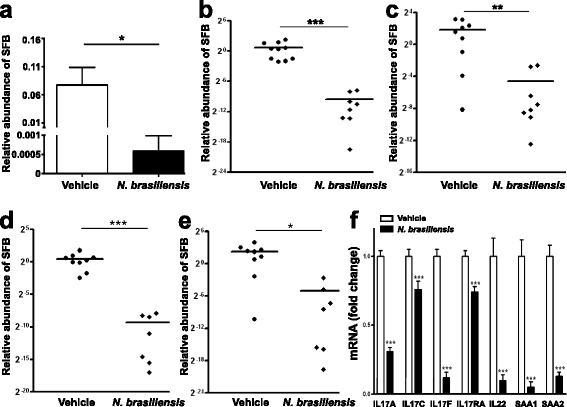
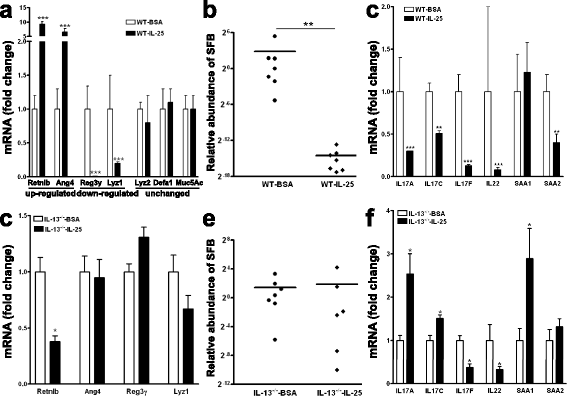
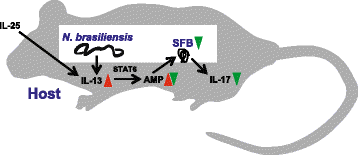
Results: We showed that the type 2 immune response to enteric Nippostrongylus brasiliensis infection in mice was associated with altered intestinal mucin and AMP expression and shifts in microbiota composition. Most strikingly, infection reduced concentrations of intestinal segmented filamentous bacteria (SFB), known inducers of T helper 17 cells, and IL-17-associated gene expression. Infected mice deficient in IL-13 or STAT6 did not reduce SFB or IL-17, and exogenous IL-25 replicated the effects of parasite infection in wild type mice.
Conclusions: Our data show that parasite infection acts through host type 2 immunity to reduce intestinal SFB and expression of IL-17, providing an example of a microbiota-dependent immune modulation by parasites.
Figures



Similar articles
-
Critical role of IL-25 in nematode infection-induced alterations in intestinal function.J Immunol. 2010 Dec 1;185(11):6921-9. doi: 10.4049/jimmunol.1000450. Epub 2010 Oct 25. J Immunol. 2010. PMID: 20974983 Free PMC article.
-
Chronic intestinal nematode infection induces Stat6-independent interleukin-5 production and causes eosinophilic inflammatory responses in mice.Immunology. 2004 Aug;112(4):615-23. doi: 10.1046/j.1365-2567.2004.01909.x. Immunology. 2004. PMID: 15270733 Free PMC article.
-
IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis.Immunity. 1998 Feb;8(2):255-64. doi: 10.1016/s1074-7613(00)80477-x. Immunity. 1998. PMID: 9492006
-
Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites.Immunol Rev. 2004 Oct;201:139-55. doi: 10.1111/j.0105-2896.2004.00192.x. Immunol Rev. 2004. PMID: 15361238 Review.
-
Segmented filamentous bacteria take the stage.Mucosal Immunol. 2010 May;3(3):209-12. doi: 10.1038/mi.2010.3. Epub 2010 Feb 10. Mucosal Immunol. 2010. PMID: 20147894 Free PMC article. Review.
Cited by
-
Guts within guts: the microbiome of the intestinal helminth parasite Ascaris suum is derived but distinct from its host.Microbiome. 2022 Dec 16;10(1):229. doi: 10.1186/s40168-022-01399-5. Microbiome. 2022. PMID: 36527132 Free PMC article.
-
Strongyle Infection and Gut Microbiota: Profiling of Resistant and Susceptible Horses Over a Grazing Season.Front Physiol. 2018 Mar 21;9:272. doi: 10.3389/fphys.2018.00272. eCollection 2018. Front Physiol. 2018. PMID: 29618989 Free PMC article.
-
Baseline Gut Microbiota Composition Is Associated With Schistosoma mansoni Infection Burden in Rodent Models.Front Immunol. 2020 Nov 18;11:593838. doi: 10.3389/fimmu.2020.593838. eCollection 2020. Front Immunol. 2020. PMID: 33329584 Free PMC article.
-
Effect of intestinal tapeworms on the gut microbiota of the common carp, Cyprinus carpio.Parasit Vectors. 2019 May 22;12(1):252. doi: 10.1186/s13071-019-3510-z. Parasit Vectors. 2019. PMID: 31113452 Free PMC article.
-
The relationships between faecal egg counts and gut microbial composition in UK Thoroughbreds infected by cyathostomins.Int J Parasitol. 2018 May;48(6):403-412. doi: 10.1016/j.ijpara.2017.11.003. Epub 2018 Feb 9. Int J Parasitol. 2018. PMID: 29432771 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

