An intact helical domain is required for Gα14 to stimulate phospholipase Cβ
- PMID: 26377666
- PMCID: PMC4573470
- DOI: 10.1186/s12900-015-0043-3
An intact helical domain is required for Gα14 to stimulate phospholipase Cβ
Abstract
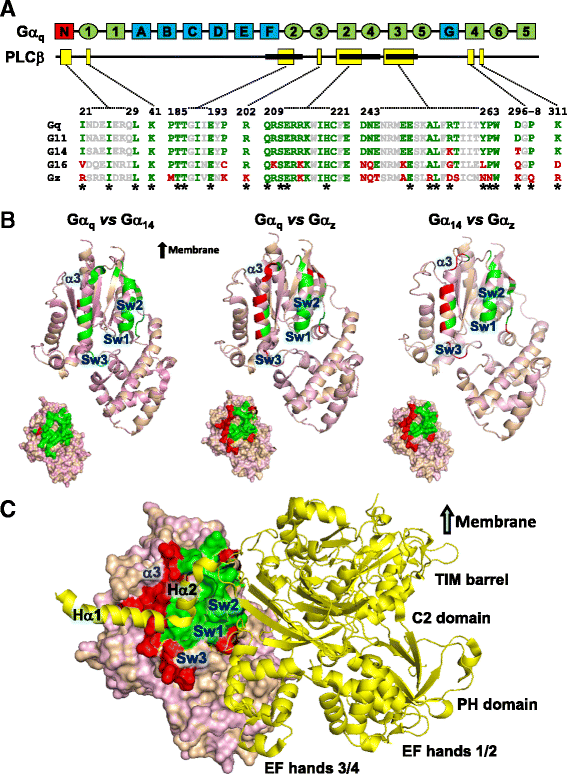
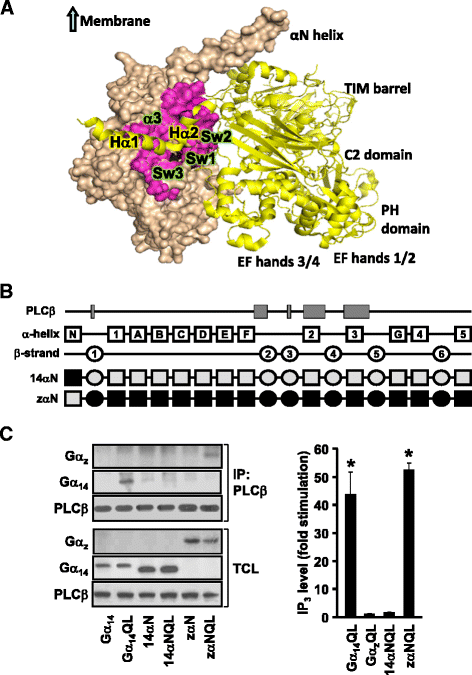
Background: Stimulation of phospholipase Cβ (PLCβ) by the activated α-subunit of Gq (Gαq) constitutes a major signaling pathway for cellular regulation, and structural studies have recently revealed the molecular interactions between PLCβ and Gαq. Yet, most of the PLCβ-interacting residues identified on Gαq are not unique to members of the Gαq family. Molecular modeling predicts that the core PLCβ-interacting residues located on the switch regions of Gαq are similarly positioned in Gαz which does not stimulate PLCβ. Using wild-type and constitutively active chimeras constructed between Gαz and Gα14, a member of the Gαq family, we examined if the PLCβ-interacting residues identified in Gαq are indeed essential.
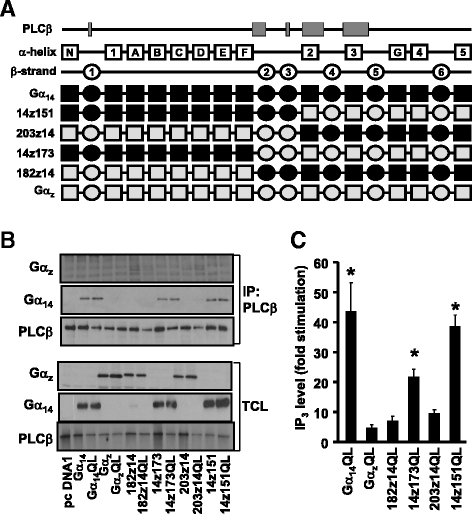
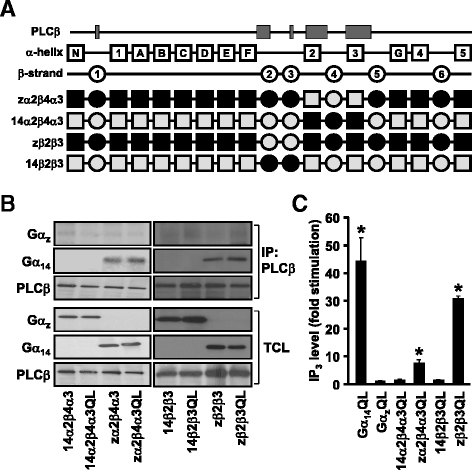
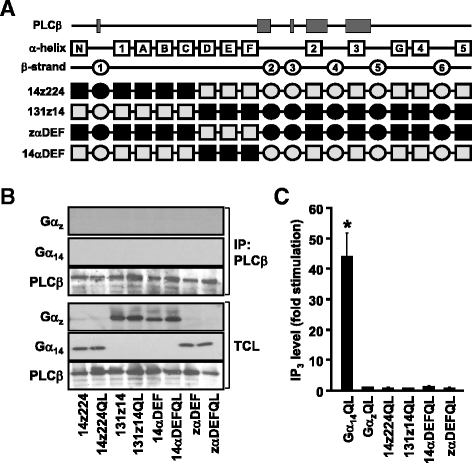
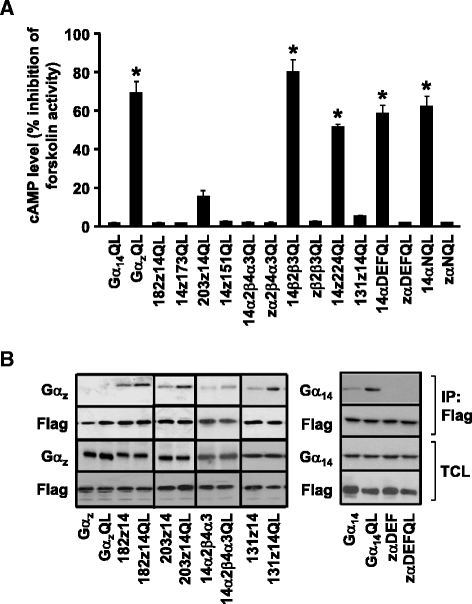
Results: Four chimeras with the core PLCβ-interacting residues composed of Gαz sequences were capable of binding PLCβ2 and stimulating the formation of inositol trisphosphate. Surprisingly, all chimeras with a Gαz N-terminal half failed to functionally associate with PLCβ2, despite the fact that many of them contained the core PLCβ-interacting residues from Gα14. Further analyses revealed that the non-PLCβ2 interacting chimeras were capable of interacting with other effector molecules such as adenylyl cyclase and tetratricopeptide repeat 1, indicating that they could adopt a GTP-bound active conformation.
Conclusion: Collectively, our study suggests that the previously identified PLCβ-interacting residues are insufficient to ensure productive interaction of Gα14 with PLCβ, while an intact N-terminal half of Gα14 is apparently required for PLCβ interaction.
Figures
References
-
- Jhon DY, Lee HH, Park D, Lee CW, Lee KH, Yoo OJ, et al. Cloning, sequencing, purification, and Gq-dependent activation of phospholipase C-beta 3. J Biol Chem. 1993;268(9):6654–6661. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

