Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity
- PMID: 26377898
- PMCID: PMC4607048
- DOI: 10.1016/j.immuni.2015.08.016
Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity
Erratum in
- Immunity. 2015 Oct 20;43(4):830
Abstract
Innate resistance to Candida albicans in mucosal tissues requires the production of interleukin-17A (IL-17A) by tissue-resident cells early during infection, but the mechanism of cytokine production has not been precisely defined. In the skin, we found that dermal γδ T cells were the dominant source of IL-17A during C. albicans infection and were required for pathogen resistance. Induction of IL-17A from dermal γδ T cells and resistance to C. albicans required IL-23 production from CD301b(+) dermal dendritic cells (dDCs). In addition, we found that sensory neurons were directly activated by C. albicans. Ablation of sensory neurons increased susceptibility to C. albicans infection, which could be rescued by exogenous addition of the neuropeptide CGRP. These data define a model in which nociceptive pathways in the skin drive production of IL-23 by CD301b(+) dDCs resulting in IL-17A production from γδ T cells and resistance to cutaneous candidiasis.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


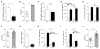

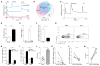
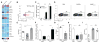
Comment in
-
IL-17A Has Some Nerve!Immunity. 2015 Sep 15;43(3):414-5. doi: 10.1016/j.immuni.2015.08.025. Immunity. 2015. PMID: 26377893
References
-
- Assas BM, Miyan JA, Pennock JL. Cross-talk between neural and immune receptors provides a potential mechanism of homeostatic regulation in the gut mucosa. Mucosal Immunol. 2014;7:1283–1289. - PubMed
-
- Bannon AW, Malmberg AB. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr Protoc Neurosci. 2007;8:9. - PubMed
-
- Bär E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity. 2014;40:117–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

