A multiprotein occupancy map of the mRNP on the 3' end of histone mRNAs
- PMID: 26377992
- PMCID: PMC4604434
- DOI: 10.1261/rna.053389.115
A multiprotein occupancy map of the mRNP on the 3' end of histone mRNAs
Abstract
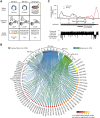
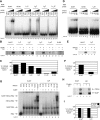
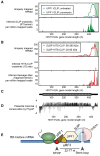
The animal replication-dependent (RD) histone mRNAs are coordinately regulated with chromosome replication. The RD-histone mRNAs are the only known cellular mRNAs that are not polyadenylated. Instead, the mature transcripts end in a conserved stem-loop (SL) structure. This SL structure interacts with the stem-loop binding protein (SLBP), which is involved in all aspects of RD-histone mRNA metabolism. We used several genomic methods, including high-throughput sequencing of cross-linked immunoprecipitate (HITS-CLIP) to analyze the RNA-binding landscape of SLBP. SLBP was not bound to any RNAs other than histone mRNAs. We performed bioinformatic analyses of the HITS-CLIP data that included (i) clustering genes by sequencing read coverage using CVCA, (ii) mapping the bound RNA fragment termini, and (iii) mapping cross-linking induced mutation sites (CIMS) using CLIP-PyL software. These analyses allowed us to identify specific sites of molecular contact between SLBP and its RD-histone mRNA ligands. We performed in vitro crosslinking assays to refine the CIMS mapping and found that uracils one and three in the loop of the histone mRNA SL preferentially crosslink to SLBP, whereas uracil two in the loop preferentially crosslinks to a separate component, likely the 3'hExo. We also performed a secondary analysis of an iCLIP data set to map UPF1 occupancy across the RD-histone mRNAs and found that UPF1 is bound adjacent to the SLBP-binding site. Multiple proteins likely bind the 3' end of RD-histone mRNAs together with SLBP.
Keywords: 3′hExo; CIMS; CLIP-seq; H2A.X; H2AFX; HITS-CLIP; RBPome; RIP-chip; RIP-seq; RNP; SLBP; UPF1; histone mRNA.
© 2015 Brooks et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures





Similar articles
-
Genome-wide analysis of mRNAs bound to the histone stem-loop binding protein.RNA. 2006 Oct;12(10):1853-67. doi: 10.1261/rna.76006. Epub 2006 Aug 24. RNA. 2006. PMID: 16931877 Free PMC article.
-
The C-terminal extension of Lsm4 interacts directly with the 3' end of the histone mRNP and is required for efficient histone mRNA degradation.RNA. 2014 Jan;20(1):88-102. doi: 10.1261/rna.042531.113. Epub 2013 Nov 19. RNA. 2014. PMID: 24255165 Free PMC article.
-
Structure of histone mRNA stem-loop, human stem-loop binding protein, and 3'hExo ternary complex.Science. 2013 Jan 18;339(6117):318-21. doi: 10.1126/science.1228705. Science. 2013. PMID: 23329046 Free PMC article.
-
Formation of the 3' end of histone mRNA.Gene. 1999 Oct 18;239(1):1-14. doi: 10.1016/s0378-1119(99)00367-4. Gene. 1999. PMID: 10571029 Review.
-
Stem-loop binding protein and metal carcinogenesis.Semin Cancer Biol. 2021 Nov;76:38-44. doi: 10.1016/j.semcancer.2021.08.006. Epub 2021 Aug 18. Semin Cancer Biol. 2021. PMID: 34416372 Free PMC article. Review.
Cited by
-
Cyclin F-Mediated Degradation of SLBP Limits H2A.X Accumulation and Apoptosis upon Genotoxic Stress in G2.Mol Cell. 2016 Nov 3;64(3):507-519. doi: 10.1016/j.molcel.2016.09.010. Epub 2016 Oct 20. Mol Cell. 2016. PMID: 27773672 Free PMC article.
-
Determining degradation intermediates and the pathway of 3' to 5' degradation of histone mRNA using high-throughput sequencing.Methods. 2019 Feb 15;155:104-115. doi: 10.1016/j.ymeth.2018.11.001. Epub 2018 Nov 5. Methods. 2019. PMID: 30408609 Free PMC article. Review.
-
Robust transcriptome-wide discovery of RNA-binding protein binding sites with enhanced CLIP (eCLIP).Nat Methods. 2016 Jun;13(6):508-14. doi: 10.1038/nmeth.3810. Epub 2016 Mar 28. Nat Methods. 2016. PMID: 27018577 Free PMC article.
-
ALYREF links 3'-end processing to nuclear export of non-polyadenylated mRNAs.EMBO J. 2019 May 2;38(9):e99910. doi: 10.15252/embj.201899910. Epub 2019 Mar 11. EMBO J. 2019. PMID: 30858280 Free PMC article.
-
Discovering sequence and structure landscapes in RNA interaction motifs.Nucleic Acids Res. 2019 Jun 4;47(10):4958-4969. doi: 10.1093/nar/gkz250. Nucleic Acids Res. 2019. PMID: 31162604 Free PMC article.
References
-
- Amrani N, Sachs MS, Jacobson A. 2006. Early nonsense: mRNA decay solves a translational problem. Nat Rev Mol Cell Biol 7: 415–425. - PubMed
-
- Braastad CD, Hovhannisyan H, van Wijnen AJ, Stein JL, Stein GS. 2004. Functional characterization of a human histone gene cluster duplication. Gene 342: 35–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
