Differential susceptibility to colorectal cancer due to naturally occurring gut microbiota
- PMID: 26378041
- PMCID: PMC4741795
- DOI: 10.18632/oncotarget.5604
Differential susceptibility to colorectal cancer due to naturally occurring gut microbiota
Abstract
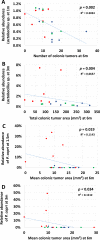
Recent studies investigating the human microbiome have identified particular bacterial species that correlate with the presence of colorectal cancer. To evaluate the role of qualitatively different but naturally occurring gut microbiota and the relationship with colorectal cancer development, genetically identical embryos from the Polyposis in Rat Colon (Pirc) rat model of colorectal cancer were transferred into recipients of three different genetic backgrounds (F344/NHsd, LEW/SsNHsd, and Crl:SD). Tumor development in the pups was tracked longitudinally via colonoscopy, and end-stage tumor burden was determined. To confirm vertical transmission and identify associations between the gut microbiota and disease phenotype, the fecal microbiota was characterized in recipient dams 24 hours pre-partum, and in Pirc rat offspring prior to and during disease progression. Our data show that the gut microbiota varies between rat strains, with LEW/SsNHsd having a greater relative abundance of the bacteria Prevotella copri. The mature gut microbiota of pups resembled the profile of their dams, indicating that the dam is the primary determinant of the developing microbiota. Both male and female F344-Pirc rats harboring the Lewis microbiota had decreased tumor burden relative to genetically identical rats harboring F344 or SD microbiota. Significant negative correlations were detected between tumor burden and the relative abundance of specific taxa from samples taken at weaning and shortly thereafter, prior to observable adenoma development. Notably, this naturally occurring variation in the gut microbiota is associated with a significant difference in severity of colorectal cancer, and the abundance of certain taxa is associated with decreased tumor burden.
Keywords: Pirc; colorectal cancer; gut; microbiota; rat.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Effect of the dietary polyacetylenes falcarinol and falcarindiol on the gut microbiota composition in a rat model of colorectal cancer.BMC Res Notes. 2018 Jun 27;11(1):411. doi: 10.1186/s13104-018-3527-y. BMC Res Notes. 2018. PMID: 29945666 Free PMC article.
-
Comparison of human gut microbiota in control subjects and patients with colorectal carcinoma in adenoma: Terminal restriction fragment length polymorphism and next-generation sequencing analyses.Oncol Rep. 2016 Jan;35(1):325-33. doi: 10.3892/or.2015.4398. Epub 2015 Nov 4. Oncol Rep. 2016. PMID: 26549775
-
Composition of the gut microbiota transcends genetic determinants of malaria infection severity and influences pregnancy outcome.EBioMedicine. 2019 Jun;44:639-655. doi: 10.1016/j.ebiom.2019.05.052. Epub 2019 May 31. EBioMedicine. 2019. PMID: 31160271 Free PMC article.
-
Can we change our microbiome to prevent colorectal cancer development?Acta Oncol. 2015;54(8):1085-95. doi: 10.3109/0284186X.2015.1054949. Epub 2015 Jun 15. Acta Oncol. 2015. PMID: 26073561 Review.
-
The interrelationships of the gut microbiome and inflammation in colorectal carcinogenesis.Clin Lab Med. 2014 Dec;34(4):699-710. doi: 10.1016/j.cll.2014.08.002. Epub 2014 Sep 15. Clin Lab Med. 2014. PMID: 25439270 Free PMC article. Review.
Cited by
-
Interactions of Segmented Filamentous Bacteria (Candidatus Savagella) and bacterial drivers in colitis-associated colorectal cancer development.PLoS One. 2020 Jul 24;15(7):e0236595. doi: 10.1371/journal.pone.0236595. eCollection 2020. PLoS One. 2020. PMID: 32706816 Free PMC article.
-
OCULAR FINDINGS AND SELECT OPHTHALMIC DIAGNOSTIC TESTS IN CAPTIVE AMERICAN WHITE PELICANS (PELECANUS ERYTHRORHYNCHOS).J Zoo Wildl Med. 2017 Sep;48(3):675-682. doi: 10.1638/2016-0256.1. J Zoo Wildl Med. 2017. PMID: 28920779 Free PMC article.
-
Increased Enterococcus faecalis infection is associated with clinically active Crohn disease.Medicine (Baltimore). 2016 Sep;95(39):e5019. doi: 10.1097/MD.0000000000005019. Medicine (Baltimore). 2016. PMID: 27684872 Free PMC article.
-
The curious case of Prevotella copri.Gut Microbes. 2023 Dec;15(2):2249152. doi: 10.1080/19490976.2023.2249152. Gut Microbes. 2023. PMID: 37655441 Free PMC article. Review.
-
Microbiota composition of simultaneously colonized mice housed under either a gnotobiotic isolator or individually ventilated cage regime.Sci Rep. 2017 Feb 7;7:42245. doi: 10.1038/srep42245. Sci Rep. 2017. PMID: 28169374 Free PMC article.
References
-
- Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, Fitzgerald GF, Deane J, O'Connor M, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. - PubMed
-
- Queipo-Ortuno MI, Boto-Ordonez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, Cardona Diaz F, Andres-Lacueva C, Tinahones FJ. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. The American journal of clinical nutrition. 2012;95:1323–1334. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

