Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties
- PMID: 26378047
- PMCID: PMC4745678
- DOI: 10.18632/oncotarget.5611
Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties
Abstract
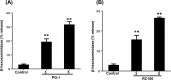
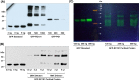
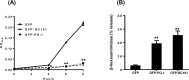
Preclinical evaluation of Retrocyclins (RC-100, RC-101) and Protegrin-1 (PG-1) antimicrobial peptides (AMPs) is important because of their therapeutic potential against bacterial, fungal and viral infections. Human mast cells (HMCs) play important roles in host defense and wound healing but the abilities of retrocyclins and protegrin-1 to harness these functions have not been investigated. Here, we report that chemically synthesized RC-100 and PG-1 caused calcium mobilization and degranulation in HMCs but these responses were not blocked by an inhibitor of formyl peptide receptor-like 1 (FPRL1), a known receptor for AMPs. However, RC-100 and PG-1 induced degranulation in rat basophilic leukemia (RBL-2H3) cells stably expressing Mas related G protein coupled receptor X2 (MrgX2). Chemical synthesis of these AMPs is prohibitively expensive and post-synthesis modifications (cyclization, disulfide bonds, folding) are inadequate for optimal antimicrobial activity. Indeed, we found that synthetic RC-100, which caused mast cell degranulation via MrgX2, did not display any antimicrobial activity. Green-fluorescent protein (GFP)-tagged RC-101 (analog of RC-100) and GFP-tagged PG-1 purified from transgenic plant chloroplasts killed bacteria and induced mast cell degranulation. Furthermore, GFP-PG1 bound specifically to RBL-2H3 cells expressing MrgX2. These findings suggest that retrocyclins and protegrins activate HMCs independently of FPRL1 but via MrgX2. Harnessing this novel feature of AMPs to activate mast cell's host defense/wound healing properties in addition to their antimicrobial activities expands their clinical potential. Low cost production of AMPs in plants should facilitate their advancement to the clinic overcoming major hurdles in current production systems.
Keywords: Immune response; Immunity; Immunology and Microbiology Section; MrgX2; antimicrobial peptides; chloroplast; mast cells; protegrin; retrocyclin.
Conflict of interest statement
All authors have no conflict of interest except HD who has several awarded US and global patents on expression of human therapeutic proteins including antimicrobial peptides in plant chloroplasts.
Figures


Similar articles
-
Modulation of host defense peptide-mediated human mast cell activation by LPS.Innate Immun. 2016 Jan;22(1):21-30. doi: 10.1177/1753425915610643. Epub 2015 Oct 28. Innate Immun. 2016. PMID: 26511058 Free PMC article.
-
Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization.J Biol Chem. 2011 Dec 30;286(52):44739-49. doi: 10.1074/jbc.M111.277152. Epub 2011 Nov 8. J Biol Chem. 2011. PMID: 22069323 Free PMC article.
-
G protein coupled receptor specificity for C3a and compound 48/80-induced degranulation in human mast cells: roles of Mas-related genes MrgX1 and MrgX2.Eur J Pharmacol. 2011 Oct 1;668(1-2):299-304. doi: 10.1016/j.ejphar.2011.06.027. Epub 2011 Jul 3. Eur J Pharmacol. 2011. PMID: 21741965 Free PMC article.
-
The Origin, Expression, Function and Future Research Focus of a G Protein-coupled Receptor, Mas-related Gene X2 (MrgX2).Prog Histochem Cytochem. 2015 Jul;50(1-2):11-7. doi: 10.1016/j.proghi.2015.06.001. Epub 2015 Jun 8. Prog Histochem Cytochem. 2015. PMID: 26106044 Review.
-
Emerging Roles for MAS-Related G Protein-Coupled Receptor-X2 in Host Defense Peptide, Opioid, and Neuropeptide-Mediated Inflammatory Reactions.Adv Immunol. 2017;136:123-162. doi: 10.1016/bs.ai.2017.06.002. Epub 2017 Jul 24. Adv Immunol. 2017. PMID: 28950944 Review.
Cited by
-
Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases.J Allergy Clin Immunol. 2016 Sep;138(3):700-710. doi: 10.1016/j.jaci.2016.04.051. Epub 2016 Jul 20. J Allergy Clin Immunol. 2016. PMID: 27448446 Free PMC article. Review.
-
Vaccination via Chloroplast Genetics: Affordable Protein Drugs for the Prevention and Treatment of Inherited or Infectious Human Diseases.Annu Rev Genet. 2016 Nov 23;50:595-618. doi: 10.1146/annurev-genet-120215-035349. Epub 2016 Oct 21. Annu Rev Genet. 2016. PMID: 27893966 Free PMC article. Review.
-
Multifaceted MRGPRX2: New insight into the role of mast cells in health and disease.J Allergy Clin Immunol. 2021 Aug;148(2):293-308. doi: 10.1016/j.jaci.2021.03.049. Epub 2021 May 4. J Allergy Clin Immunol. 2021. PMID: 33957166 Free PMC article. Review.
-
Modulation of host defense peptide-mediated human mast cell activation by LPS.Innate Immun. 2016 Jan;22(1):21-30. doi: 10.1177/1753425915610643. Epub 2015 Oct 28. Innate Immun. 2016. PMID: 26511058 Free PMC article.
-
Angiogenic Host Defense Peptide AG-30/5C and Bradykinin B2 Receptor Antagonist Icatibant Are G Protein Biased Agonists for MRGPRX2 in Mast Cells.J Immunol. 2019 Feb 15;202(4):1229-1238. doi: 10.4049/jimmunol.1801227. Epub 2019 Jan 16. J Immunol. 2019. PMID: 30651343 Free PMC article.
References
-
- Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, et al. Antibiotic resistance-the need for global solutions. The Lancet Infectious diseases. 2013;13:1057–1098. - PubMed
-
- Zaga-Clavellina V, Ruiz M, Flores-Espinosa P, Vega-Sanchez R, Flores-Pliego A, Estrada-Gutierrez G, Sosa-Gonzalez I, Morales-Mendez I, Osorio-Caballero M. Tissue-specific human beta-defensins (HBD)-1, HBD-2 and HBD-3 secretion profile from human amniochorionic membranes stimulated with Candida albicans in a two-compartment tissue culture system. Reproductive biology and endocrinology: RB&E. 2012;10:70. - PMC - PubMed
-
- van der Does AM, Bergman P, Agerberth B, Lindbom L. Induction of the human cathelicidin LL-37 as a novel treatment against bacterial infections. J Leukoc Biol. 2012;92:735–742. - PubMed
-
- Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, Oppenheim JJ. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

