Dengue Virus NS1 Protein Modulates Cellular Energy Metabolism by Increasing Glyceraldehyde-3-Phosphate Dehydrogenase Activity
- PMID: 26378175
- PMCID: PMC4645330
- DOI: 10.1128/JVI.01342-15
Dengue Virus NS1 Protein Modulates Cellular Energy Metabolism by Increasing Glyceraldehyde-3-Phosphate Dehydrogenase Activity
Abstract
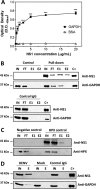
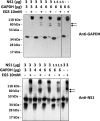
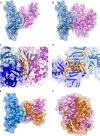
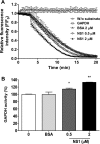
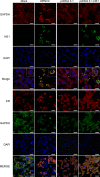
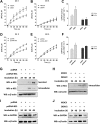
Dengue is one of the main public health concerns worldwide. Recent estimates indicate that over 390 million people are infected annually with the dengue virus (DENV), resulting in thousands of deaths. Among the DENV nonstructural proteins, the NS1 protein is the only one whose function during replication is still unknown. NS1 is a 46- to 55-kDa glycoprotein commonly found as both a membrane-associated homodimer and a soluble hexameric barrel-shaped lipoprotein. Despite its role in the pathogenic process, NS1 is essential for proper RNA accumulation and virus production. In the present study, we identified that glyceraldehyde-3-phosphate dehydrogenase (GAPDH) interacts with intracellular NS1. Molecular docking revealed that this interaction occurs through the hydrophobic protrusion of NS1 and the hydrophobic residues located at the opposite side of the catalytic site. Moreover, addition of purified recombinant NS1 enhanced the glycolytic activity of GAPDH in vitro. Interestingly, we observed that DENV infection promoted the relocalization of GAPDH to the perinuclear region, where NS1 is commonly found. Both DENV infection and expression of NS1 itself resulted in increased GAPDH activity. Our findings indicate that the NS1 protein acts to increase glycolytic flux and, consequently, energy production, which is consistent with the recent finding that DENV induces and requires glycolysis for proper replication. This is the first report to propose that NS1 is an important modulator of cellular energy metabolism. The data presented here provide new insights that may be useful for further drug design and the development of alternative antiviral therapies against DENV.
Importance: Dengue represents a serious public health problem worldwide and is caused by infection with dengue virus (DENV). Estimates indicate that half of the global population is at risk of infection, with almost 400 million cases occurring per year. The NS1 glycoprotein is found in both the intracellular and the extracellular milieus. Despite the fact that NS1 has been commonly associated with DENV pathogenesis, it plays a pivotal but unknown role in the replication process. In an effort to understand the role of intracellular NS1, we demonstrate that glyceraldehyde-3-phosphate dehydrogenase (GAPDH) interacts with NS1. Our results indicate that NS1 increases the glycolytic activity of GAPDH in vitro. Interestingly, the GAPDH activity was increased during DENV infection, and NS1 expression alone was sufficient to enhance intracellular GAPDH activity in BHK-21 cells. Overall, our findings suggest that NS1 is an important modulator of cellular energy metabolism by increasing glycolytic flux.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi:10.1038/nature12060. - DOI - PMC - PubMed
-
- Gutsche I, Coulibaly F, Voss JE, Salmon J, d'Alayer J, Ermonval M, Larquet E, Charneau P, Krey T, Megret F, Guittet E, Rey FA, Flamand M. 2011. Secreted dengue virus nonstructural protein NS1 is an atypical barrel-shaped high-density lipoprotein. Proc Natl Acad Sci U S A 108:8003–8008. doi:10.1073/pnas.1017338108. - DOI - PMC - PubMed
-
- Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. 2002. Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol 40:376–381. doi:10.1128/JCM.40.02.376-381.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

