Automatic reconstruction of physiological gestures used in a model of birdsong production
- PMID: 26378204
- PMCID: PMC4737419
- DOI: 10.1152/jn.00385.2015
Automatic reconstruction of physiological gestures used in a model of birdsong production
Abstract
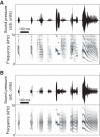
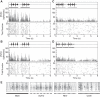
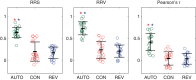
Highly coordinated learned behaviors are key to understanding neural processes integrating the body and the environment. Birdsong production is a widely studied example of such behavior in which numerous thoracic muscles control respiratory inspiration and expiration: the muscles of the syrinx control syringeal membrane tension, while upper vocal tract morphology controls resonances that modulate the vocal system output. All these muscles have to be coordinated in precise sequences to generate the elaborate vocalizations that characterize an individual's song. Previously we used a low-dimensional description of the biomechanics of birdsong production to investigate the associated neural codes, an approach that complements traditional spectrographic analysis. The prior study used algorithmic yet manual procedures to model singing behavior. In the present work, we present an automatic procedure to extract low-dimensional motor gestures that could predict vocal behavior. We recorded zebra finch songs and generated synthetic copies automatically, using a biomechanical model for the vocal apparatus and vocal tract. This dynamical model described song as a sequence of physiological parameters the birds control during singing. To validate this procedure, we recorded electrophysiological activity of the telencephalic nucleus HVC. HVC neurons were highly selective to the auditory presentation of the bird's own song (BOS) and gave similar selective responses to the automatically generated synthetic model of song (AUTO). Our results demonstrate meaningful dimensionality reduction in terms of physiological parameters that individual birds could actually control. Furthermore, this methodology can be extended to other vocal systems to study fine motor control.
Keywords: bird's own song; dynamical systems; modeling software; peripheral vocal production model; vocal learning.
Copyright © 2015 the American Physiological Society.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

