Layer-specific modulation of entorhinal cortical excitability by presubiculum in a rat model of temporal lobe epilepsy
- PMID: 26378210
- PMCID: PMC4737416
- DOI: 10.1152/jn.00823.2015
Layer-specific modulation of entorhinal cortical excitability by presubiculum in a rat model of temporal lobe epilepsy
Abstract
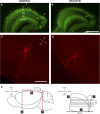
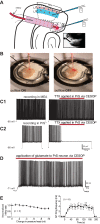
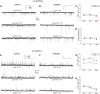
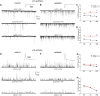
Temporal lobe epilepsy (TLE) is the most common form of epilepsy in adults and is often refractory to antiepileptic medications. The medial entorhinal area (MEA) is affected in TLE but mechanisms underlying hyperexcitability of MEA neurons require further elucidation. Previous studies suggest that inputs from the presubiculum (PrS) contribute to MEA pathophysiology. We assessed electrophysiologically how PrS influences MEA excitability using the rat pilocarpine model of TLE. PrS-MEA connectivity was confirmed by electrically stimulating PrS afferents while recording from neurons within superficial layers of MEA. Assessment of alterations in PrS-mediated synaptic drive to MEA neurons was made following focal application of either glutamate or NBQX to the PrS in control and epileptic animals. Here, we report that monosynaptic inputs to MEA from PrS neurons are conserved in epileptic rats, and that PrS modulation of MEA excitability is layer-specific. PrS contributes more to synaptic inhibition of LII stellate cells than excitation. Under epileptic conditions, stellate cell inhibition is significantly reduced while excitatory synaptic drive is maintained at levels similar to control. PrS contributes to both synaptic excitation and inhibition of LIII pyramidal cells in control animals. Under epileptic conditions, overall excitatory synaptic drive to these neurons is enhanced while inhibitory synaptic drive is maintained at control levels. Additionally, neither glutamate nor NBQX applied focally to PrS now affected EPSC and IPSC frequency of LIII pyramidal neurons. These layer-specific changes in PrS-MEA interactions are unexpected and of significance in unraveling pathophysiological mechanisms underlying TLE.
Keywords: electrophysiology; glutamate application; hyperexcitability; presubiculum; temporal lobe epilepsy.
Copyright © 2015 the American Physiological Society.
Figures


References
-
- Abbasi S, Kumar SS. Electrophysiological and morphological characterization of cells in superficial layers of rat presubiculum. J Comp Neurol 521: 3116–3132, 2013. - PubMed
-
- Bartesaghi R, Di Maio V, Gessi T. Topographic activation of the medial entorhinal cortex by presubicular commissural projections. J Comp Neurol 487: 283–299, 2005. - PubMed
-
- Bartolomei F, Khalil M, Wendling F, Sontheimer A, Regis J, Ranjeva JP, Guye M, Chauvel P. Entorhinal cortex involvement in human mesial temporal lobe epilepsy: an electrophysiologic and volumetric study. Epilepsia 46: 677–687, 2005. - PubMed
-
- Bear J, Fountain NB, Lothman EW. Responses of the superficial entorhinal cortex in vitro in slices from naive and chronically epileptic rats. J Neurophysiol 76: 2928–2940, 1996. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

