Methods for identification and confirmation of targeted subgroups in clinical trials: A systematic review
- PMID: 26378339
- PMCID: PMC4732423
- DOI: 10.1080/10543406.2015.1092034
Methods for identification and confirmation of targeted subgroups in clinical trials: A systematic review
Abstract
Important objectives in the development of stratified medicines include the identification and confirmation of subgroups of patients with a beneficial treatment effect and a positive benefit-risk balance. We report the results of a literature review on methodological approaches to the design and analysis of clinical trials investigating a potential heterogeneity of treatment effects across subgroups. The identified approaches are classified based on certain characteristics of the proposed trial designs and analysis methods. We distinguish between exploratory and confirmatory subgroup analysis, frequentist, Bayesian and decision-theoretic approaches and, last, fixed-sample, group-sequential, and adaptive designs and illustrate the available trial designs and analysis strategies with published case studies.
Keywords: Enrichment design; personalized medicine; precision medicine; predictive biomarkers; subgroup identification.
Figures
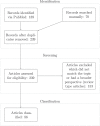

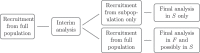
References
-
- Alosh M., Huque M.F. A flexible strategy for testing subgroups and overall population. Statistics in Medicine. 2009;28:3–23. - PubMed
-
- Alosh M., Huque M.F. Multiplicity considerations for subgroup analysis subject to consistency constraint: Multiplicity considerations for subgroup analysis. Biometrical Journal. 2013;55:444–462. - PubMed
-
- Bang Y.J., Van Cutsem E., Feyereislova A., Chung H.C., Shen L., Sawaki A., Lordick F., Ohtsu A., Omuro Y., Satoh T., Aprile G., Kulikov E., Hill J., Lehle M., Rüschoff J., Kang Y.K. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of her2-positive advanced gastric or gastro-oesophageal junction cancer (toga): a phase 3, open-label, randomised controlled trial. The Lancet. 2010;367:687–698. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
