Exploiting Expression of Hippo Effector, Yap, for Expansion of Functional Islet Mass
- PMID: 26378466
- PMCID: PMC4627601
- DOI: 10.1210/me.2014-1375
Exploiting Expression of Hippo Effector, Yap, for Expansion of Functional Islet Mass
Abstract
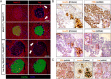
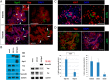
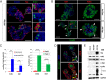
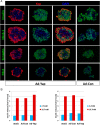
Loss of pancreas β-cell function is the precipitating factor in all forms of diabetes. Cell replacement therapies, such as islet transplantation, remain the best hope for a cure; however, widespread implementation of this method is hampered by availability of donor tissue. Thus, strategies that expand functional β-cell mass are crucial for widespread usage in diabetes cell replacement therapy. Here, we investigate the regulation of the Hippo-target protein, Yes-associated protein (Yap), during development of the endocrine pancreas and its function after reactivation in human cadaveric islets. Our results demonstrate that Yap expression is extinguished at the mRNA level after neurogenin-3-dependent specification of the pancreas endocrine lineage, correlating with proliferation decreases in these cells. Interestingly, when a constitutively active form of Yap was expressed in human cadaver islets robust increases in proliferation were noted within insulin-producing β-cells. Importantly, proliferation in these cells occurs without negatively affecting β-cell differentiation or functional status. Finally, we show that the proproliferative mammalian target of rapamycin pathway is activated after Yap expression, providing at least one explanation for the observed increases in β-cell proliferation. Together, these results provide a foundation for manipulating Yap activity as a novel approach to expand functional islet mass for diabetes regenerative therapy.
Figures



Similar articles
-
Proproliferative and antiapoptotic action of exogenously introduced YAP in pancreatic β cells.JCI Insight. 2016 Nov 3;1(18):e86326. doi: 10.1172/jci.insight.86326. JCI Insight. 2016. PMID: 27812538 Free PMC article.
-
Hippo pathway-mediated YAP1/TAZ inhibition is essential for proper pancreatic endocrine specification and differentiation.Elife. 2024 Jul 25;13:e84532. doi: 10.7554/eLife.84532. Elife. 2024. PMID: 39051998 Free PMC article.
-
Emerging role of Hippo signalling in pancreatic biology: YAP re-expression and plausible link to islet cell apoptosis and replication.Biochimie. 2017 Feb;133:56-65. doi: 10.1016/j.biochi.2016.12.009. Epub 2016 Dec 18. Biochimie. 2017. PMID: 28003126 Review.
-
Hippo signaling regulates pancreas development through inactivation of Yap.Mol Cell Biol. 2012 Dec;32(24):5116-28. doi: 10.1128/MCB.01034-12. Epub 2012 Oct 15. Mol Cell Biol. 2012. PMID: 23071096 Free PMC article.
-
PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration.Stem Cell Res Ther. 2017 Nov 2;8(1):240. doi: 10.1186/s13287-017-0694-z. Stem Cell Res Ther. 2017. PMID: 29096722 Free PMC article. Review.
Cited by
-
VGLL4 and MENIN function as TEAD1 corepressors to block pancreatic β cell proliferation.Cell Rep. 2023 Jan 31;42(1):111904. doi: 10.1016/j.celrep.2022.111904. Epub 2023 Jan 19. Cell Rep. 2023. PMID: 36662616 Free PMC article.
-
Regulation of autophagy by perilysosomal calcium: a new player in β-cell lipotoxicity.Exp Mol Med. 2024 Feb;56(2):273-288. doi: 10.1038/s12276-024-01161-x. Epub 2024 Feb 1. Exp Mol Med. 2024. PMID: 38297165 Free PMC article. Review.
-
TEAD1 regulates cell proliferation through a pocket-independent transcription repression mechanism.Nucleic Acids Res. 2022 Dec 9;50(22):12723-12738. doi: 10.1093/nar/gkac1063. Nucleic Acids Res. 2022. PMID: 36484096 Free PMC article.
-
Replicative capacity of β-cells and type 1 diabetes.J Autoimmun. 2016 Jul;71:59-68. doi: 10.1016/j.jaut.2016.03.014. Epub 2016 Apr 28. J Autoimmun. 2016. PMID: 27133598 Free PMC article. Review.
-
Proproliferative and antiapoptotic action of exogenously introduced YAP in pancreatic β cells.JCI Insight. 2016 Nov 3;1(18):e86326. doi: 10.1172/jci.insight.86326. JCI Insight. 2016. PMID: 27812538 Free PMC article.
References
-
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

