Home Use of an Artificial Beta Cell in Type 1 Diabetes
- PMID: 26379095
- PMCID: PMC4697362
- DOI: 10.1056/NEJMoa1509351
Home Use of an Artificial Beta Cell in Type 1 Diabetes
Abstract
Background: The feasibility, safety, and efficacy of prolonged use of an artificial beta cell (closed-loop insulin-delivery system) in the home setting have not been established.
Methods: In two multicenter, crossover, randomized, controlled studies conducted under free-living home conditions, we compared closed-loop insulin delivery with sensor-augmented pump therapy in 58 patients with type 1 diabetes. The closed-loop system was used day and night by 33 adults and overnight by 25 children and adolescents. Participants used the closed-loop system for a 12-week period and sensor-augmented pump therapy (control) for a similar period. The primary end point was the proportion of time that the glucose level was between 70 mg and 180 mg per deciliter for adults and between 70 mg and 145 mg per deciliter for children and adolescents.
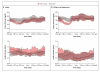
Results: Among adults, the proportion of time that the glucose level was in the target range was 11.0 percentage points (95% confidence interval [CI], 8.1 to 13.8) greater with the use of the closed-loop system day and night than with control therapy (P<0.001). The mean glucose level was lower during the closed-loop phase than during the control phase (difference, -11 mg per deciliter; 95% CI, -17 to -6; P<0.001), as were the area under the curve for the period when the glucose level was less than 63 mg per deciliter (39% lower; 95% CI, 24 to 51; P<0.001) and the mean glycated hemoglobin level (difference, -0.3%; 95% CI, -0.5 to -0.1; P=0.002). Among children and adolescents, the proportion of time with the nighttime glucose level in the target range was higher during the closed-loop phase than during the control phase (by 24.7 percentage points; 95% CI, 20.6 to 28.7; P<0.001), and the mean nighttime glucose level was lower (difference, -29 mg per deciliter; 95% CI, -39 to -20; P<0.001). The area under the curve for the period in which the day-and-night glucose levels were less than 63 mg per deciliter was lower by 42% (95% CI, 4 to 65; P=0.03). Three severe hypoglycemic episodes occurred during the closed-loop phase when the closed-loop system was not in use.
Conclusions: Among patients with type 1 diabetes, 12-week use of a closed-loop system, as compared with sensor-augmented pump therapy, improved glucose control, reduced hypoglycemia, and, in adults, resulted in a lower glycated hemoglobin level. (Funded by the JDRF and others; AP@home04 and APCam08 ClinicalTrials.gov numbers, NCT01961622 and NCT01778348.).
Figures

References
-
- McKnight JA, Wild SH, Lamb MJ, et al. Glycaemic control of Type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med. 2015;32:1036–50. - PubMed
-
- Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care. 1997;20:22–5. - PubMed
-
- Johnson SR, Cooper MN, Davis EA, Jones TW. Hypoglycaemia, fear of hypoglycaemia and quality of life in children with Type 1 diabetes and their parents. Diabet Med. 2013;30:1126–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
