Acinetobacter baumannii Virulence Is Mediated by the Concerted Action of Three Phospholipases D
- PMID: 26379240
- PMCID: PMC4574555
- DOI: 10.1371/journal.pone.0138360
Acinetobacter baumannii Virulence Is Mediated by the Concerted Action of Three Phospholipases D
Abstract
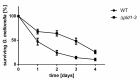
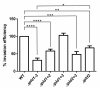
Acinetobacter baumannii causes a broad range of opportunistic infections in humans. Its success as an emerging pathogen is due to a combination of increasing antibiotic resistance, environmental persistence and adaptation to the human host. To date very little is known about the molecular basis of the latter. Here we demonstrate that A. baumannii can use phosphatidylcholine, an integral part of human cell membranes, as sole carbon and energy source. We report on the identification of three phospholipases belonging to the PLD superfamily. PLD1 and PLD2 appear restricted to the bacteria and display the general features of bacterial phospholipases D. They possess two PLDc_2 PFAM domains each encompassing the HxKx4Dx6GS/GGxN (HKD) motif necessary for forming the catalytic core. The third candidate, PLD3, is found in bacteria as well as in eukaryotes and harbours only one PLDc_2 PFAM domain and one conserved HKD motif, which however do not overlap. Employing a markerless mutagenesis system for A. baumannii ATCC 19606T, we generated a full set of PLD knock-out mutants. Galleria mellonella infection studies as well as invasion experiments using A549 human lung epithelial cells revealed that the three PLDs act in a concerted manner as virulence factors and are playing an important role in host cell invasion.
Conflict of interest statement
Figures




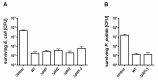

References
-
- Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii . Nat Rev Microbiol. 2007;5: 939–951. - PubMed
-
- Gaynes R, Edwards JR, System NNIS. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41: 848–854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

