A Mitochondrion-Targeted Antioxidant Ameliorates Isoflurane-Induced Cognitive Deficits in Aging Mice
- PMID: 26379247
- PMCID: PMC4575031
- DOI: 10.1371/journal.pone.0138256
A Mitochondrion-Targeted Antioxidant Ameliorates Isoflurane-Induced Cognitive Deficits in Aging Mice
Abstract
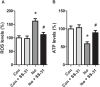
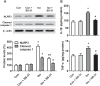
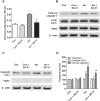
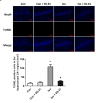
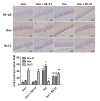
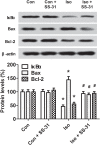
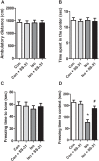
Isoflurane possesses neurotoxicity and can induce cognitive deficits, particularly in aging mammals. Mitochondrial reactive oxygen species (mtROS) have been linked to the early pathogenesis of this disorder. However, the role of mtROS remains to be evaluated due to a lack of targeted method to treat mtROS. Here, we determined in aging mice the effects of the mitochondrion-targeted antioxidant SS-31, on cognitive deficits induced by isoflurane, a general inhalation anesthetic. We further investigated the possible mechanisms underlying the effects of SS-31 on hippocampal neuro-inflammation and apoptosis. The results showed that isoflurane induced hippocampus-dependent memory deficit, which was associated with mitochondrial dysfunction including reduced ATP contents, increased ROS levels, and mitochondrial swelling. Treatment with SS-31 significantly ameliorated isoflurane-induced cognitive deficits through the improvement of mitochondrial integrity and function. Mechanistically, SS-31 treatment suppressed pro-inflammatory responses by decreasing the levels of NF-κB, NLRP3, caspase 1, IL-1β, and TNF-α; and inhibited the apoptotic pathway by decreasing the Bax/Bcl-2 ratio, reducing the release of cytochrome C, and blocking the cleavage of caspase 3. Our results indicate that isoflurane-induced cognitive deficits may be attenuated by mitochondrion-targeted antioxidants, such as SS-31. Therefore, SS-31 may have therapeutic potentials in preventing injuries from oxidative stresses that contribute to anesthetic-induced neurotoxicity.
Conflict of interest statement
Figures

References
-
- Loop T, Dovi-Akue D, Frick M, Roesslein M, Egger L, Humar M, et al. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102(6):1147–57. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

