Blockade of pathological retinal ganglion cell hyperactivity improves optogenetically evoked light responses in rd1 mice
- PMID: 26379501
- PMCID: PMC4548307
- DOI: 10.3389/fncel.2015.00330
Blockade of pathological retinal ganglion cell hyperactivity improves optogenetically evoked light responses in rd1 mice
Abstract
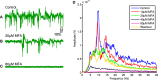
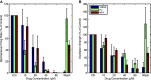
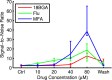
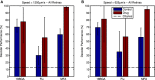
Retinitis pigmentosa (RP) is a progressive retinal dystrophy that causes visual impairment and eventual blindness. Retinal prostheses are the best currently available vision-restoring treatment for RP, but only restore crude vision. One possible contributing factor to the poor quality of vision achieved with prosthetic devices is the pathological retinal ganglion cell (RGC) hyperactivity that occurs in photoreceptor dystrophic disorders. Gap junction blockade with meclofenamic acid (MFA) was recently shown to diminish RGC hyperactivity and improve the signal-to-noise ratio (SNR) of RGC responses to light flashes and electrical stimulation in the rd10 mouse model of RP. We sought to extend these results to spatiotemporally patterned optogenetic stimulation in the faster-degenerating rd1 model and compare the effectiveness of a number of drugs known to disrupt rd1 hyperactivity. We crossed rd1 mice with a transgenic mouse line expressing the light-sensitive cation channel channelrhodopsin2 (ChR2) in RGCs, allowing them to be stimulated directly using high-intensity blue light. We used 60-channel ITO multielectrode arrays to record ChR2-mediated RGC responses from wholemount, ex-vivo retinas to full-field and patterned stimuli before and after application of MFA, 18-β-glycyrrhetinic acid (18BGA, another gap junction blocker) or flupirtine (Flu, a Kv7 potassium channel opener). All three drugs decreased spontaneous RGC firing, but 18BGA and Flu also decreased the sensitivity of RGCs to optogenetic stimulation. Nevertheless, all three drugs improved the SNR of ChR2-mediated responses. MFA also made it easier to discern motion direction of a moving bar from RGC population responses. Our results support the hypothesis that reduction of pathological RGC spontaneous activity characteristic in retinal degenerative disorders may improve the quality of visual responses in retinal prostheses and they provide insights into how best to achieve this for optogenetic prostheses.
Keywords: 18-beta-glycyrrhetinic acid; flupirtine; meclofenamic acid; optogenetics; retinal degeneration; retinal prosthesis; spontaneous activity.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

