LGR4 and Its Role in Intestinal Protection and Energy Metabolism
- PMID: 26379625
- PMCID: PMC4548225
- DOI: 10.3389/fendo.2015.00131
LGR4 and Its Role in Intestinal Protection and Energy Metabolism
Abstract
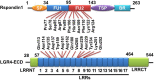
Leucine-rich repeat-containing G protein-coupled receptors were identified by the unique nature of their long leucine-rich repeat extracellular domains. Distinct from classical G protein-coupled receptors which act via G proteins, LGR4 functions mainly through Wnt/β-catenin signaling to regulate cell proliferation, differentiation, and adult stem cell homeostasis. LGR4 is widely expressed in tissues ranging from the reproductive system, urinary system, sensory organs, digestive system, and the central nervous system, indicating LGR4 may have multiple functions in development. Here, we focus on the digestive system by reviewing its effects on crypt cells differentiation and stem cells maintenance, which are important for cell regeneration after injury. Through effects on Wnt/β-catenin signaling and cell proliferation, LGR4 and its endogenous ligands, R-spondins, are involved in colon tumorigenesis. LGR4 also contributes to regulation of energy metabolism, including food intake, energy expenditure, and lipid metabolism, as well as pancreatic β-cell proliferation and insulin secretion. This review summarizes the identification of LGR4, its endogenous ligand, ligand-receptor binding and intracellular signaling. Physiological functions include intestinal development and energy metabolism. The potential effects of LGR4 and its ligand in the treatment of inflammatory bowel disease, chemoradiotherapy-induced gut damage, colorectal cancer, and diabetes are also discussed.
Keywords: LGR4; R-spondin; colon cancer; diabetes; digestive system; energy metabolism.
Figures

Similar articles
-
Crystal structure of LGR4-Rspo1 complex: insights into the divergent mechanisms of ligand recognition by leucine-rich repeat G-protein-coupled receptors (LGRs).J Biol Chem. 2015 Jan 23;290(4):2455-65. doi: 10.1074/jbc.M114.599134. Epub 2014 Dec 5. J Biol Chem. 2015. PMID: 25480784 Free PMC article.
-
Aberrantly expressed LGR4 empowers Wnt signaling in multiple myeloma by hijacking osteoblast-derived R-spondins.Proc Natl Acad Sci U S A. 2017 Jan 10;114(2):376-381. doi: 10.1073/pnas.1618650114. Epub 2016 Dec 27. Proc Natl Acad Sci U S A. 2017. PMID: 28028233 Free PMC article.
-
The Role of LGR4 (GPR48) in Normal and Cancer Processes.Int J Mol Sci. 2021 Apr 29;22(9):4690. doi: 10.3390/ijms22094690. Int J Mol Sci. 2021. PMID: 33946652 Free PMC article. Review.
-
R-Spondin potentiates Wnt/β-catenin signaling through orphan receptors LGR4 and LGR5.PLoS One. 2012;7(7):e40976. doi: 10.1371/journal.pone.0040976. Epub 2012 Jul 16. PLoS One. 2012. PMID: 22815884 Free PMC article.
-
Role of Leucine-Rich Repeat-Containing G-Protein-Coupled Receptors 4-6 (LGR4-6) in the Ovary and Other Female Reproductive Organs: A Literature Review.Cell Transplant. 2025 Jan-Dec;34:9636897241303441. doi: 10.1177/09636897241303441. Cell Transplant. 2025. PMID: 39874091 Free PMC article. Review.
Cited by
-
LGR5 and LGR6 in stem cell biology and ovarian cancer.Oncotarget. 2017 Aug 11;9(1):1346-1355. doi: 10.18632/oncotarget.20178. eCollection 2018 Jan 2. Oncotarget. 2017. PMID: 29416699 Free PMC article. Review.
-
Regeneration of thyroid follicles from primordial cells in a murine thyroidectomized model.Lab Invest. 2017 Apr;97(4):478-489. doi: 10.1038/labinvest.2016.158. Epub 2017 Jan 23. Lab Invest. 2017. PMID: 28112758 Free PMC article.
-
High Expression of CD200 and CD200R1 Distinguishes Stem and Progenitor Cell Populations within Mammary Repopulating Units.Stem Cell Reports. 2018 Jul 10;11(1):288-302. doi: 10.1016/j.stemcr.2018.05.013. Epub 2018 Jun 21. Stem Cell Reports. 2018. PMID: 29937142 Free PMC article.
-
MiR-449a regulates caprine endometrial stromal cell apoptosis and endometrial receptivity.Sci Rep. 2017 Sep 25;7(1):12248. doi: 10.1038/s41598-017-12451-y. Sci Rep. 2017. PMID: 28947781 Free PMC article.
-
Reduction of specific enterocytes from loss of intestinal LGR4 improves lipid metabolism in mice.Nat Commun. 2024 May 23;15(1):4393. doi: 10.1038/s41467-024-48622-5. Nat Commun. 2024. PMID: 38782937 Free PMC article.
References
-
- Luo CW, Hsueh AJ. Genomic analyses of the evolution of LGR genes. Chang Gung Med J (2006) 29:2–8. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

