Cell aggregation promotes pyoverdine-dependent iron uptake and virulence in Pseudomonas aeruginosa
- PMID: 26379660
- PMCID: PMC4552172
- DOI: 10.3389/fmicb.2015.00902
Cell aggregation promotes pyoverdine-dependent iron uptake and virulence in Pseudomonas aeruginosa
Abstract
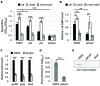
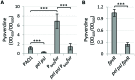
In Pseudomonas aeruginosa the Gac signaling system and the second messenger cyclic diguanylate (c-di-GMP) participate in the control of the switch between planktonic and biofilm lifestyles, by regulating the production of the two exopolysaccharides Pel and Psl. The Gac and c-di-GMP regulatory networks also coordinately promote the production of the pyoverdine siderophore, and the extracellular polysaccharides Pel and Psl have recently been found to mediate c-di-GMP-dependent regulation of pyoverdine genes. Here we demonstrate that Pel and Psl are also essential for Gac-mediated activation of pyoverdine production. A pel psl double mutant produces very low levels of pyoverdine and shows a marked reduction in the expression of the pyoverdine-dependent virulence factors exotoxin A and PrpL protease. While the exopolysaccharide-proficient parent strain forms multicellular planktonic aggregates in liquid cultures, the Pel and Psl-deficient mutant mainly grows as dispersed cells. Notably, artificially induced cell aggregation is able to restore pyoverdine-dependent gene expression in the pel psl mutant, in a way that appears to be independent of iron diffusion or siderophore signaling, as well as of recently described contact-dependent mechanosensitive systems. This study demonstrates that cell aggregation represents an important cue triggering the expression of pyoverdine-related genes in P. aeruginosa, suggesting a novel link between virulence gene expression, cell-cell interaction and the multicellular community lifestyle.
Keywords: Pseudomonas aeruginosa; cell aggregates; extracellular polysaccharide; gene regulation; iron uptake; mechanosensor; siderophore; virulence.
Figures



Similar articles
-
The Gac/Rsm and cyclic-di-GMP signalling networks coordinately regulate iron uptake in Pseudomonas aeruginosa.Environ Microbiol. 2014 Mar;16(3):676-88. doi: 10.1111/1462-2920.12164. Epub 2013 Jun 25. Environ Microbiol. 2014. PMID: 23796404
-
Matrix Polysaccharides and SiaD Diguanylate Cyclase Alter Community Structure and Competitiveness of Pseudomonas aeruginosa during Dual-Species Biofilm Development with Staphylococcus aureus.mBio. 2018 Nov 6;9(6):e00585-18. doi: 10.1128/mBio.00585-18. mBio. 2018. PMID: 30401769 Free PMC article.
-
Multiple diguanylate cyclase-coordinated regulation of pyoverdine synthesis in Pseudomonas aeruginosa.Environ Microbiol Rep. 2015 Jun;7(3):498-507. doi: 10.1111/1758-2229.12278. Epub 2015 Apr 8. Environ Microbiol Rep. 2015. PMID: 25683454
-
Recent perspectives on the molecular basis of biofilm formation by Pseudomonas aeruginosa and approaches for treatment and biofilm dispersal.Folia Microbiol (Praha). 2018 Jul;63(4):413-432. doi: 10.1007/s12223-018-0585-4. Epub 2018 Jan 19. Folia Microbiol (Praha). 2018. PMID: 29352409 Review.
-
Aspergillus-Pseudomonas interaction, relevant to competition in airways.Med Mycol. 2019 Apr 1;57(Supplement_2):S228-S232. doi: 10.1093/mmy/myy087. Med Mycol. 2019. PMID: 30816973 Review.
Cited by
-
Pseudomonas aeruginosa Strains from Both Clinical and Environmental Origins Readily Adopt a Stable Small-Colony-Variant Phenotype Resulting from Single Mutations in c-di-GMP Pathways.J Bacteriol. 2022 Oct 18;204(10):e0018522. doi: 10.1128/jb.00185-22. Epub 2022 Sep 14. J Bacteriol. 2022. PMID: 36102640 Free PMC article.
-
Role of Iron Uptake Systems in Pseudomonas aeruginosa Virulence and Airway Infection.Infect Immun. 2016 Jul 21;84(8):2324-2335. doi: 10.1128/IAI.00098-16. Print 2016 Aug. Infect Immun. 2016. PMID: 27271740 Free PMC article.
-
Interaction between Pseudomonas aeruginosa and Aspergillus fumigatus in cystic fibrosis.PeerJ. 2018 Nov 9;6:e5931. doi: 10.7717/peerj.5931. eCollection 2018. PeerJ. 2018. PMID: 30430043 Free PMC article.
-
The RNA Chaperone Protein Hfq Regulates the Characteristic Sporulation and Insecticidal Activity of Bacillus thuringiensis.Front Microbiol. 2022 Apr 11;13:884528. doi: 10.3389/fmicb.2022.884528. eCollection 2022. Front Microbiol. 2022. PMID: 35479624 Free PMC article.
-
Interbacterial Antagonism Mediated by a Released Polysaccharide.J Bacteriol. 2022 May 17;204(5):e0007622. doi: 10.1128/jb.00076-22. Epub 2022 Apr 21. J Bacteriol. 2022. PMID: 35446119 Free PMC article.
References
-
- Barton H. A., Johnson Z., Cox C. D., Vasil A. I., Vasil M. L. (1996). Ferric uptake regulator mutants of Pseudomonas aeruginosa with distinct alterations in the iron-dependent repression of exotoxin A and siderophores in aerobic and microaerobic environments. Mol. Microbiol. 21 1001–1017. 10.1046/j.1365-2958.1996.381426.x - DOI - PubMed
-
- Brencic A., McFarland K. A., McManus H. R., Castang S., Mogno I., Dove S. L., et al. (2009). The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol. Microbiol. 73 434–445. 10.1111/j.1365-2958.2009.06782.x - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

