Proton therapy for pancreatic cancer
- PMID: 26380057
- PMCID: PMC4569591
- DOI: 10.4251/wjgo.v7.i9.141
Proton therapy for pancreatic cancer
Abstract
Radiotherapy is commonly offered to patients with pancreatic malignancies although its ultimate utility is compromised since the pancreas is surrounded by exquisitely radiosensitive normal tissues, such as the duodenum, stomach, jejunum, liver, and kidneys. Proton radiotherapy can be used to create dose distributions that conform to tumor targets with significant normal tissue sparing. Because of this, protons appear to represent a superior modality for radiotherapy delivery to patients with unresectable tumors and those receiving postoperative radiotherapy. A particularly exciting opportunity for protons also exists for patients with resectable and marginally resectable disease. In this paper, we review the current literature on proton therapy for pancreatic cancer and discuss scenarios wherein the improvement in the therapeutic index with protons may have the potential to change the management paradigm for this malignancy.
Keywords: Pancreatic cancer; Proton therapy; Review.
Figures


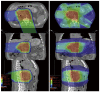
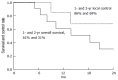
References
-
- Paganetti H. Proton Therapy Physics. Boca Raton, FL: CRC Press;; 2011.
-
- Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. - PubMed
-
- Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–1585. - PubMed
-
- Abrams RA, Lillemoe KD, Piantadosi S. Continuing controversy over adjuvant therapy of pancreatic cancer. Lancet. 2001;358:1565–1566. - PubMed
-
- Hammel P, Huguet F, Van Laethem JL. Comparison of chemoradiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC) controlled after 4 months of gemcitabine with or without erlotinib: Final results of the international phase III LAP 07 study [abstract] J Clin Oncol. 2013;31:LBA4003a.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

